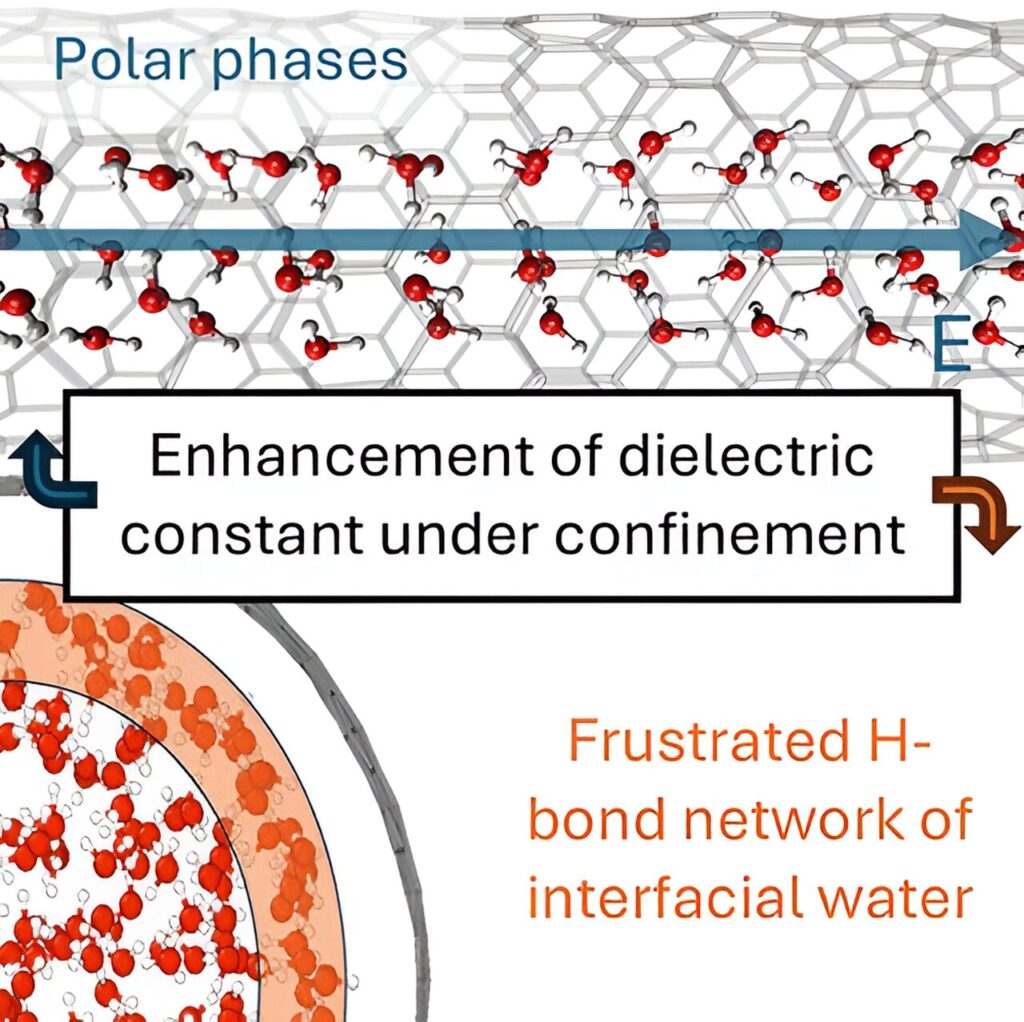
When water gets inside nanopores with sizes below 10 nanometers, new physics emerge: new phases of ice were observed and ultrafast proton transport was measured. Confined water also plays a role in biology, where aquaporins cross cellular membranes to allow specific transport of water and other small molecules through nanometer-scale channels.
However, this field lacks a fundamental understanding of how confinement impacts the ability of water to screen an electric field inside one-dimensional pores.
To solve this challenge, Lawrence Livermore National Laboratory (LLNL) scientists and a collaborator at University of Texas at Austin turned to simulations to explain the first-order response of confined water to applied electric fields. The research appears on the cover of The Journal of Physical Chemistry Letters.
The authors have found an increase in the ability of water to screen electric fields applied along the axis of the one-dimensional nanopore. This enhancement arises from a longer-range alignment of water dipoles under confinement relative to the bulk fluid, leading even to the formation of exotic phases of water (ferroelectric ices) under extreme confinement.
“It’s necessary to understand the ability of the confined liquid to screen electric fields and how this varies from the bulk environment,” said LLNL scientist Marcos Calegari Andrade, lead author of the paper. “An improved understanding of the dielectric response of confined water is important not only for advancing separation technologies but also for other emerging applications, such as energy storage and conversion.”
Nanopores smaller than 10 nanometers have shown promising ion selectivity for applications ranging from water desalination to devices used for photochemical water splitting.
“Fundamental studies of the confinement effect on water’s dielectric constant are beneficial to understand and improve current technologies,” said LLNL scientist Anh Pham, co-author of the paper.
In the new research, the team looked to explore the computational efficiency of machine learning (ML) to derive the hydrophobic nanoconfinement effect on the dielectric properties of water from a first-principles-based approach. The ML allowed the team to predict the potential energy surface of the system as well as the molecular dipole of water, both with the accuracy of quantum mechanical calculations.
“Our work reveals peculiar impacts of 1-D hydrophobic nanoconfinement, not only on the dielectric constant, but also on the electronic structure of water that cannot be observed with simulations based on conventional parametric force fields,” Calegari Andrade said.