Industry has long relied upon energy-intensive processes, such as distillation and crystallization, to separate molecules that ultimately serve as ingredients in medicine, chemicals and other products.
In recent decades, there has been a push to supplant these processes with membranes, which are potentially a lower-cost and eco-friendly alternative. Unfortunately, most membranes are made from polymers that degrade during use, making them impractical.
To solve this problem, a University at Buffalo-led research team has created a new, sturdier membrane that can withstand harsh environments—high temperatures, high pressure and complex chemical solvents—associated with industrial separation processes.
Made from an inorganic material called carbon-doped metal oxide, it is described in a study published Sept. 7 in Science.
“The processes of separating molecules—whether for water desalination, the production of medicine or fertilizers—use an incredible amount of energy,” says the study’s corresponding author, Miao Yu, Ph.D., SUNY Empire Innovation Professor in the Department of Chemical and Biological Engineering in the University at Buffalo School of Engineering and Applied Sciences.
“What we have developed is a technique to easily fabricate defect-free, strong membranes that have rigid nanopores that can be precisely controlled to allow different-sized molecules to pass through,” adds Yu, a core faculty member in the UB RENEW Institute.
The study’s first authors are Bratin Sengupta, a Ph.D. student in Yu’s lab, and Qiaobei Dong, Ph.D., who studied under Yu and now works at GTI Energy.
Inspired by semiconductors
To create the membrane, the research team took inspiration from two common, but unrelated, manufacturing techniques.
The first is molecular layer deposition, which involves layering thin films of materials and is most often associated with semiconductor production. The second technique is interfacial polymerization, which is a method of combining chemicals that is commonly used to create fuel cells, chemical sensors and other electronics.
“These methods are not new,” says Sengupta, “however the manner in which we apply them is, and that is the key to creating our new nanoporous membranes.”
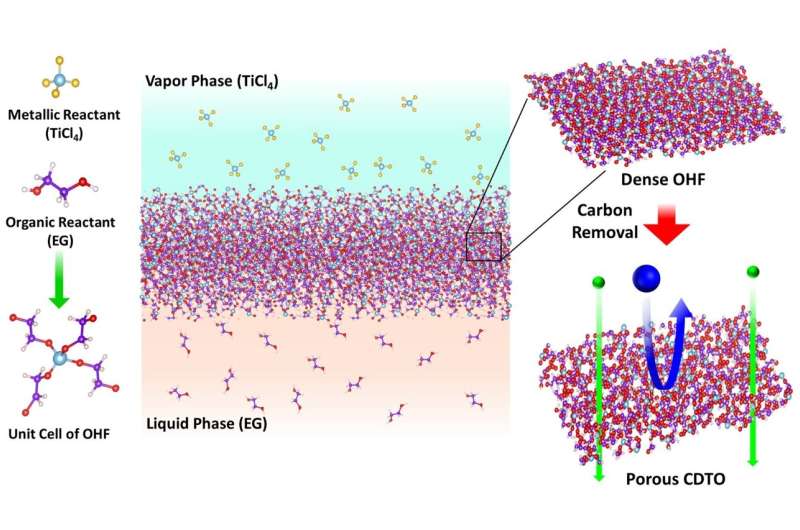
In experiments, researchers merged two low-cost reactants—liquid ethylene glycol and gaseous titanium tetrachloride—on an aluminum-based support. Within minutes, the reaction created a thin-film.
To create the nanopores, they applied heat to the film. The heat burns off carbon, creating tiny, microscopic holes for molecules to pass through. The size of the nanopores can be anywhere from 0.6 to 1.2 nanometers in diameter—as determined by the calcination gas environment, as well as the amount and duration of heat.
The method allows researchers to avoid a nagging problem—small holes merging into larger ones, thus making them more porous than intended—with creating polymer-based membranes.
Potential to reduce carbon footprint
The new membrane can withstand temperatures up to 284°F (140°C) and pressures up to 30 atmospheres when exposed to organic solvents. These attributes are key because they allow the membrane to separate molecules at high temperatures (for most polymer membranes to work, the temperature of the solvents must be lowered, which is costly from an energy standpoint).
“From this point of view, our membrane has the potential reduce the carbon footprint of many industrial processes,” Yu says.
To demonstrate the membrane’s effectiveness, the team showed it could separate boscalid, a fungicide used to protect crops, from its catalyst and starting reagent. The entire process occurred at 194°F.
The team is planning additional experiments to prove the membrane is capable of being scaled up for commercial products. Additionally, Yu plans to start a company to further the technology’s commercial viability.