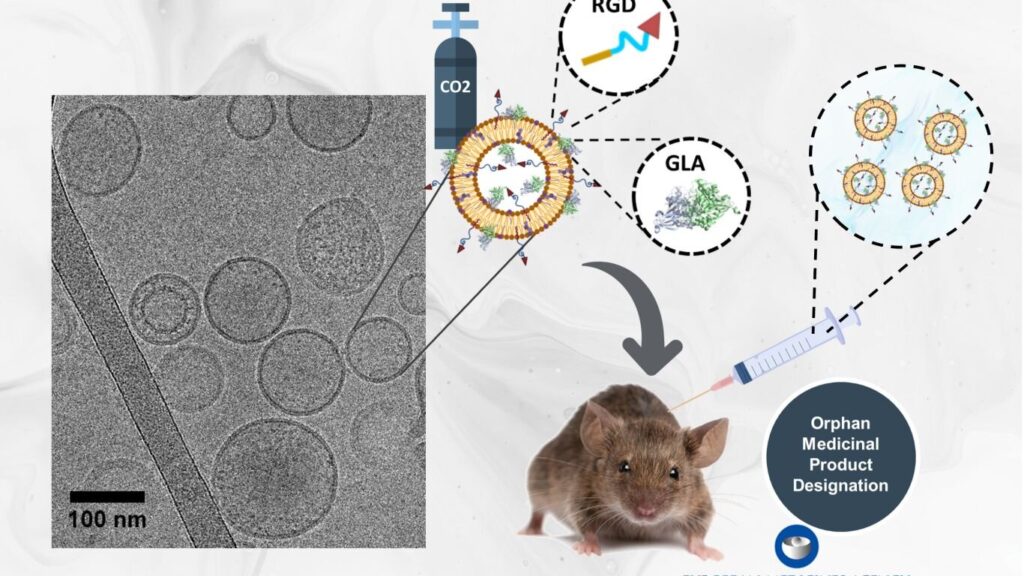
An international research team has developed a new therapy based on nanotechnology called nanoGLA for the treatment of Fabry disease. The new therapeutic solution has shown remarkable efficacy in preclinical studies. The study was published this December in Science Advances.
Fabry disease is a rare genetic disorder caused by a deficiency of the enzyme GLA (alpha-galactosidase A), which leads to the accumulation of fatty substrates (mainly globotriaosylceramide or Gb3) in cells, with severe effects on various organs.
The nanoGLA therapy, based on the use of peptide-targeted nanoliposomes, effectively delivers the deficient enzyme GLA, encapsulated in the nanoliposomes, to the organs most affected by this disease. The researchers have managed to produce nanoGLA with the necessary quality and quantity for preclinical testing, as well as for advancing into clinical phases.
In studies with mouse models of Fabry disease, nanoGLA demonstrated improved efficacy compared to therapies using the unencapsulated enzyme, showing effectiveness in affected organs, including the brain, a key milestone that current therapies do not achieve. These results highlight the potential of nanoGLA to address both the systemic and cerebrovascular manifestations of Fabry disease.
In recognition of the importance of this innovation, the European Medicines Agency granted nanoGLA orphan drug designation (Orphan Medicinal Product Designation) in 2021, a crucial step in driving its development.
Elisabet González, ICMAB researcher and one of the lead authors of the article, explains, “The new nanoGLA formulation represents a promising opportunity for Fabry disease patients, especially in addressing the neurological manifestations of the disease, a limitation that current therapies cannot overcome. Our goal is to develop safer and more effective treatments by harnessing the potential of nanotechnology.”
These results have greenlighted the continued pharmaceutical development of nanoGLA toward clinical phases with human patients.