Over the past few decades, there have been significant strides in producing metal nanomaterials. Most recently, controlled synthesis has become versatile enough to regulate the exact number of atoms and ligands of very small metal nanoparticles, referred to as “clusters.”
Unlike larger metal nanoparticles or bulk metal, these clusters have intriguing optical properties like strong photoluminescence. This luminescence stems from specific electronic energy transitions available only in these incredibly tiny particles.
As such, clusters act more like molecules, standing apart from other metal forms, forming a distinctive link between singular atoms and well-understood bulk materials.
Due to their small size, the electronic and optical traits of clusters are greatly influenced by the number of metal atoms at their core and the chosen coordinating ligand.
The ability to tune the size and composition of these clusters presents numerous opportunities for their use in fluorescence, including biological imaging. Moreover, their long-lasting excited states make them an appealing category for light absorption in energy-related applications.
Photovoltaic Properties of Gold Clusters
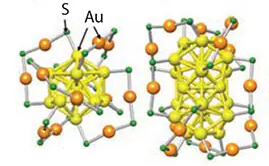
One commonly studied class of clusters is thiolate-protected gold clusters, with the molecular formula AuxSRy. Diverse versions of these clusters have been created by altering the metal atom counts (x) and using different kinds of coordinating thiols (y).
For instance, the most commonly produced/studied cluster, Au25SR18 (25 gold atoms and 18 thiol ligands), has its structure well-defined, alongside other sizes like Au38SR24 and Au144SR60, established through single crystal X-Ray diffraction (XRD). Au144SR60 displays molecule-like electronic properties with discrete electronic states.
Above this size, however, the number of atoms in the core is so large that their discrete electronic states are not distinguishable, and they behave as a metal with a full band structure. The optical absorption of particles larger than Au144SR60 is dominated by plasmon absorptions arising from collective oscillations of loosely bound electrons.
The photophysical traits of atomically precise clusters heavily rely on the specific count of metal atoms. A recent study demonstrated the substantial impact of atom count on sunlight absorption and its conversion to energy in photovoltaics.3
Beyond light absorption, understanding the behavior of excited states is crucial in determining a material’s potential for light harvesting.
Exploration into the optical properties and behavior of these clusters is still in its infancy. Gaining a deeper understanding of their characteristics holds huge potential when it comes to unlocking new opportunities for the discovery and optimization of materials specifically tailored for light-harvesting applications.
Unlike larger metal nanoparticles, thiolated gold clusters do not exhibit plasmon resonance. Their light absorption occurs within the visible spectrum. As the gold atom count rises, absorption extends into the visible range.
These clusters possess high photoactivity and sustain an extended excited state lifetime (~780 ns).2 Smaller clusters are dominated by ligand-to-metal charge complexes in their excited state, while larger ones (e.g., Au25GSH18) show core transitions affecting the excited state decay, consequently shortening the excited state lifetime.
For a more detailed photophysical characterization of these gold clusters, further information can be found elsewhere.3
The rich photochemistry of glutathione-capped gold clusters has enabled a new class of photosensitizers. Light harvesting in these gold clusters is made possible through the transfer of electrons to molecular species like methyl viologen.
Merck has used transient absorption spectroscopy to establish the mechanistic and kinetic aspects of the photoinduced electron transfer processes in suspensions of these gold clusters.2–3
A significant application of these gold clusters lies in light-to-energy conversion. Their photosensitizing abilities have also recently been explored by affixing them to mesoscopic titanium dioxide (TiO2) films.
TiO2 mesoscopic films modified with Aux-SH respond favorably to visible light when used as the photoanode in liquid-junction solar cells.
The photocurrent response aligns closely with the absorption pattern of Aux-SH clusters when metal cluster sensitized solar cells (MCSC) are combined with cadmium sulfide (CdS)-based liquid-junction solar cells.
At 400–425 nm, a maximum incident photon-to-photocurrent efficiency (IPCE) of 70% indicates effective photon capture by gold clusters, efficiently converting them into electrical energy. These MCSCs demonstrate a power conversion efficiency range of 2.03–2.36%, comparable to CdS quantum dot solar cells.
Gold cluster-sensitized solar cells manifest relatively high open-circuit voltages (0.85–0.90 V) compared to other sensitizing dyes. Merck has made use of gold nanoclusters to further boost the photovoltage of dye-sensitized solar cells employing a squaraine dye (Prod. No. 757233) and ruthenium polypyridyl complex as photosensitizer.5
Co-anchoring gold clusters on TiO2 (Prod. Nos. 799289, 204757, 232033, and 248576) alongside other sensitizing dyes results in an approximate 100 mV increase. This enhancement arises from a shift in the Fermi level towards more negative potentials due to electron accumulation in metal cluster co-sensitized solar cells.
Another intriguing trait of gold clusters is their redox capability. Cyclic voltammetry experiments reveal a broad range for reversible reduction [E0= -0.63 V vs. RHE (reversible hydrogen electrode)] and oxidation (E0= 0.97 V and 1.51 V vs. RHE) processes.
This property has been explored further to produce hydrogen in both photoelectrochemical cells and photocatalytic slurry systems. Attaching gold clusters to TiO2 particles enables electron injection into TiO2 under visible light irradiation.
These injected electrons are then transferred to an active platinum catalyst site, reducing H+ ions into hydrogen. Detailed information regarding the mechanisms of solar fuel generation is available elsewhere.6
Summary
This article has presented key insights into gold atom nanoclusters. The excellent photosensitizing ability of gold clusters renders them promising for light energy harvesting applications. Additionally, their high photoactivity, stability in aqueous medium, and biocompatibility make these clusters suitable as biotags or biomarkers.7
Given their novelty, further exploration is still necessary before their potential can be explored in energy generation and biological applications. As the scientific community ventures into designing metal clusters with customized ligands, there is much scope for additional applications of these clusters.
Acknowledgments
The research discussed here received support from the Division of Chemical Sciences, Geosciences, and Biosciences, Office of Basic Energy Sciences of the U.S. Department of Energy, under award DE-FC02-04ER15533. This work is contribution number NDRL No. 5082 from the Notre Dame Radiation Laboratory.
References
- Kurashige W, Niihori Y, Sharma S, Negishi Y. 2014. Recent Progress in the Functionalization Methods of Thiolate-Protected Gold Clusters. J. Phys. Chem. Lett.. 5(23):4134-4142. https://doi.org/10.1021/jz501941p
- Stamplecoskie KG, Chen Y, Kamat PV. 2014. Excited-State Behavior of Luminescent Glutathione-Protected Gold Clusters. J. Phys. Chem. C. 118(2):1370-1376. https://doi.org/10.1021/jp410856h
- Stamplecoskie KG, Kamat PV. 2014. Size-Dependent Excited State Behavior of Glutathione-Capped Gold Clusters and Their Light-Harvesting Capacity. J. Am. Chem. Soc.. 136(31):11093-11099. https://doi.org/10.1021/ja505361n
- Chen Y, Choi H, Kamat PV. 2013. Metal-Cluster-Sensitized Solar Cells. A New Class of Thiolated Gold Sensitizers Delivering Efficiency Greater Than 2%. J. Am. Chem. Soc.. 135(24):8822-8825. https://doi.org/10.1021/ja403807f
- Choi H, Chen Y, Stamplecoskie KG, Kamat PV. 2015. Boosting the Photovoltage of Dye-Sensitized Solar Cells with Thiolated Gold Nanoclusters. J. Phys. Chem. Lett.. 6(1):217-223. https://doi.org/10.1021/jz502485w
- Chen Y, Kamat PV. 2014. Glutathione-Capped Gold Nanoclusters as Photosensitizers. Visible Light-Induced Hydrogen Generation in Neutral Water. J. Am. Chem. Soc.. 136(16):6075-6082. https://doi.org/10.1021/ja5017365
- Retnakumari A, Setua S, Menon D, Ravindran P, Muhammed H, Pradeep T, Nair S, Koyakutty M. 2010. Molecular-receptor-specific, non-toxic, near-infrared-emitting Au cluster-protein nanoconjugates for targeted cancer imaging. Nanotechnology. 21(5):055103. https://doi.org/10.1088/0957-4484/21/5/055103
This information has been sourced, reviewed and adapted from materials provided by Merck.
For more information on this source, please visit Merck.