In energy storage and electrocatalysis, correlating electrochemical activity with nanostructured electrochemical interfaces (electrodes)1 is considered the holy grail.
It is difficult to analyze the local structure-activity relationship for these interfaces, or to measure the heterogeneity of electrode structures when employing traditional macroscopic electrochemical techniques.
This is because macroscopic electrochemical investigations can only quantify the total electron transfer on a full sample. A novel strategy for the characterization of nanoscale electrochemical activity is required to solve this challenge.
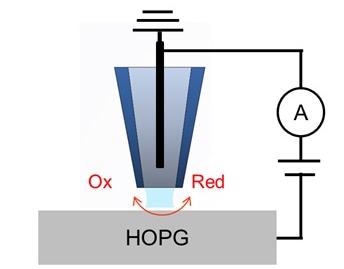
Scanning electrochemical cell microscopy (SECCM) is a novel pipette-based nanoelectrochemical scanning probe technique devised to study the local electrochemical features of electrode surfaces1-4.
The nanopipette is filled with an electroactive species and a quasi-reference counter electrode (QRCE) is inserted. When the AFM Z scanner is used to lower the nanopipette, a meniscus at the contact surface is created which triggers the formation of a tiny droplet, or nanoelectrochemical cell.
When the working electrode is positioned on the XY scanner and a bias is applied between the QRCE, the electroactive species in the confined droplet exhibit an electrochemical reaction. The collection of several cyclic voltammograms at different positions enables an electrochemical current mapping to be extracted.
Researchers in SECCM can carry out thousands of confined nanoelectrochemical measurements (where the droplet area varies from nm2 to µm2) on one surface5. It is possible to achieve high-throughput experiments.
By merely swapping a new pipette with a different electroactive species, scientists can simply modify the chemical systems and there is minimal requirement for samples to be specially prepared.
The preparation of the pipettes is cost-effective and simple. The data is simple to analyze where a higher current corresponds to an electrochemical reaction in the probed region or a higher rate.
All of the benefits outlined above make SECCM the ideal system for the electrochemical investigation of specific platinum nanoparticles3 or to correlate local electrocatalytic activities with the local structures on polycrystalline electrode surfaces5-6.
In this investigation, Park NX12 is used to analyze the electrochemically reversible [Ru(NH3)6]3+/2+ redox process at a highly-ordered pyrolytic graphite (HOPG) surface. SECCM can be used on all NX systems. A glass nanopipette with a Ag/AgCl QRCE is used in the study.
Employing previous successful experience in commercialized pipette-based electrochemical microscopy7, the software and hardware from Park Systems deliver localized nanoscopic cyclic voltammetry measurements every time that the meniscus makes contact with the surface.
Thus, a spatially resolved surface electroactivity mapping of HOPG with high throughput at the nano- and microscale is produced by the Park NX12.
This research shows the efficacy of the commercial SECCM solution from Park Systems for quantitative electroanalysis at the nanoscale. This feature could also enable the rational design of functional electromaterials with possible applications in corrosion research and energy storage (battery) investigations.
Experimental
Hexaammineruthenium (III) chloride ([Ru(NH3)6]Cl3, Sigma-Aldrich) and Potassium chloride (KCl, Sigma-Aldrich) are utilized without alterations. 0.037 g KCl and 0.0155 g [Ru(NH3)6]Cl3 are dissolved together in 10 mL deionized (DI) water to create the mixed electrolyte solution with a concentration of 50 mM KCl and 5 mM [Ru(NH3)6]Cl3.
The working electrode is the highly-ordered pyrolytic graphite (HOPG) sample which was cleaved utilizing the ‘scotch tape technique’ prior to usage8.
Decoupled XY and Z piezoelectric scanners direct the sample and pipette movement for all SECCM studies involving the Park NX12 system. The Park Systems SmartScanTM software is used to conduct the SECCM experiments.
A schematic diagram of the SECCM is displayed in Figure 1. An aqueous electrolyte solution (5 mM [Ru(NH3)6]Cl3 +50 mM KCl) is firstly used to fill a glass nanopipette. The nanopipette has a ∼100 nm-diameter tip opening which is created by pulling a borosilicate capillary.
To function as the QRCE, an Ag/AgCl electrode is injected into the pipette. The electrolyte-filled pipette then is secured on the Park SICM head and is moved above the HOPG electrode surface. A current amplifier is included in the SICM head, which is configured onto the Park NX12 system for the measurement of current.
Figure 1. Schematic illustration of SECCM configuration with a single channel pipette. Image Credit: Park Systems
Five panels and windows are included in the SECCM mode which enable the operator to manage the nanoelectrochemical process (Figure 2). A liquid meniscus is created when the pipette approaches the sample in the first stage, which functions as a nanoscale electrochemical cell.
The operator can monitor the distance from the end of the tip to the surface of the sample by utilizing the vision view (Figure 2A). A potential bias of (-0.5 V) is applied to the surface of the sample during the approach.
The current throughout the meniscus is recorded by the current log channel (Figure 2B). Variations in this current are used as signals to direct the movement of the Z scanner until a meniscus is created with no substrate contact.
The Z scanner stops when the probe meniscus is in contact with the surface, and a reduction reaction occurs inside of the confined droplet at the predetermined potential9. The current at pA range is identified by contrasting it to the background fA current in the air.
Shown in Figure 2B, the current log channel suggested that this fast change in current triggered a current spike. This relates to the variation in current versus time in the current log file when a reduction reaction occurs and the meniscus develops.
The operator can quantify the electrochemical activity at the designated position after the pipette has made contact with the droplet utilizing I/V spectroscopy mode to acquire a single linear, cyclic voltammogram.
In the control panel (Figure 2C), the operator can enter the required conditions of the experiment, such as output channels, cycle repetitions, sweep rate/speed and sample bias voltage.
When a sample bias voltage ranging from -0.5 V to 0 V is applied, the cyclic voltammograms (CVs) for the Ru (NH3)6]3+/2+ redox reaction are taken on the HOPG surface at different scan rates.
The operator can utilize the ‘Point List’ function in the Position area (Figure 2D) to assign the location for the single CV curve to be collected. The ‘Point Grid’ function enables the operator to acquire the I/V spectroscopy in repetition throughout a predefined surface and can produce an image, of the electrochemical activity.
The Approach-Retract-Scanning (ARS) mode is the term for this function10. Linear CVs are captured when each meniscus is formed while the pipette lands at different predetermined grid positions using AFM. The ‘Data View’ panel is where the obtained CV will be presented (Figure 2E).

Figure 2. SECCM Mode in Park SmartScanTM software. (A) Vision & Monitoring view. (B) Current monitor panel. (C) I/V spectroscopy parameter control panel. (D) Spectroscopy positions control panel for point list or point grid function. (E) Data viewing panel. Image Credit: Park Systems
Results and Discussion
In these SECCM studies, a glass nanopipette with a ∼100 nm-diameter tip opening filled with 5 mM [Ru(NH3)6]Cl3 is fixed on the SICM head aligned above the surface of the HOPG electrode. A linear sweep voltammetry (LSV) is employed to measure the localized electrochemical activity throughout the HOPG surface upon formation of the meniscus.
An LSV is performed in the range 0 V → -0.5 V → 0 V to gain a microscopic understanding of the [Ru (NH3)6]3+/2+ electron transfer process on HOPG. This bias range was determined by the bulk macroscopical Ru (NH3)6]3+/2+ redox reaction CV curve.
A standard SECCM LSV curve for the reduction of (NH3)6]3+ is depicted in Figure 3. The sigmoidal smooth wave shape that can be seen is typical of the LSV recorded in SECCM systems11. This sigmoidal plot relates to a quasi-steady-state voltammogram, and the steady-state limit current is approximately -5.3 pA with a -0.5 V sample voltage.
The effectiveness of Park Systems’ low-noise current detector is demonstrated by the small current magnitude detected. The redox reaction can be reversed. The [Ru(NH3)6]3+ reduction arises when the potential sweeps from 0 V to – 0.5 V, and oxidation occurs when the potential sweeps back to 0 V from -0.5 V.
![Single SECCM LSV acquired with a glass nanopipette filled with 5 mM [Ru(NH3)6]Cl3. The LSV is recorded at a sweep rate of 10 mV/s with an initial potential at 0 V.](https://d1otjdv2bf0507.cloudfront.net/images/Article_Images/ImageForArticle_5645_16119818727616650.jpg)
Figure 3. Single SECCM LSV acquired with a glass nanopipette filled with 5 mM [Ru(NH3)6]Cl3. The LSV is recorded at a sweep rate of 10 mV/s with an initial potential at 0 V. Image Credit: Park Systems
![Four overlaid SECCM LSVs in 5mM [Ru(NH3)6]3+ and 50 mM KCl at a sweep rate of 200 mV/s.](https://d1otjdv2bf0507.cloudfront.net/images/Article_Images/ImageForArticle_5645_16119818884073447.jpg)
Figure 4. Four overlaid SECCM LSVs in 5 mM [Ru(NH3)6]3+ and 50 mM KCl at a sweep rate of 200 mV/s. Image Credit: Park Systems
The SECCM LSV is robust and highly reproducible. Figure 4 depicts a series of 4 standard LSV curves acquired on HOPG at an increased scan rate of 200 mV/s. The consecutive CVs show little variation.
The limit current value (-4.6 pA) is generally similar to the one outlined in Figure 3, which suggests the nanoelectrode CV response is independent to the scan rate.
This creates the slightly widening curve at the left bottom of Figure 4 because the system cannot be considered as a true steady state. This is caused by the higher charging current with the higher scan rate.
Voltammetry measurements are taken at a range of points utilizing ARS mode to analyze the position-dependent electrochemical behavior. In brief, after the first LSV is acquired, the nanopipette is vertically retracted by several micrometers higher than the first approached position on the surface by the Z scanner. The XY scanner then travels horizontally to a predefined distance.
The Z scanner lowers repeatedly until electrical contact is made with the surface. A second LSV curve is acquired, and the process is repeated several times throughout the preset scanning region.
As a proof-of-concept, 10 LSVs were acquired at 10 adjacent positions to produce an image of the electrochemical activity and were depicted through the electrochemical current map.
Figure 5A presents a SECCM current map acquired by plotting the current which was analyzed at fixed potential of −0.5 V vs. Ag/AgCl. In this image, the color contrast relates to the current variation within ~1 pA range for the generated 10 LSV across the scan area. Figure 5B and 5C depict the individual full CV curve at position 1 and position 2.
The similarity in the EC response is caused by the fact that the nanopipette has landed on a basal surface rather than a step edge2. Additional systematic studies have been scheduled to identify the relationship between surface structures and the electrochemical activity.
![(A) Electrochemical current maps at -0.5 V vs Ag/AgCl on HOPG surface. Image is generated from a series of LSVs taken with the same nanopipette filled with 5mM [Ru(NH3)6]3+ and 50 mM KCl. The scan rate is 10 mV/s with the bias voltage starting from 0 and swept to -0.5 V vs Ag/AgCl. (B) & (C) Individual LSVs taken at position 1 and 2.](https://d1otjdv2bf0507.cloudfront.net/images/Article_Images/ImageForArticle_5645_16119819045011851.jpg)
Figure 5. (A) Electrochemical current maps at -0.5 V vs Ag/AgCl on HOPG surface. Image is generated from a series of LSVs taken with the same nanopipette filled with 5 mM [Ru(NH3)6]3+ and 50 mM KCl. The scan rate is 10 mV/s with the bias voltage starting from 0 and swept to -0.5 V vs Ag/AgCl. (B) & (C) Individual LSVs taken at position 1 and 2. Image Credit: Park Systems
Conclusion
This article demonstrates the simplicity of the first commercial SECCM in evaluating the nanoscale electroactivity mapping through the use of the advanced SmartScanTM software and the Park NX12 system.
An electrochemically reversible [Ru(NH3)6]3+/2+ redox process at the HOPG surface is measured with robustness and high reproducibility, with a current limit as small as several pA.
ARS mode is used to demonstrate the position dependence electrochemical current mapping. The results indicate that SECCM is the most recent development in nanoscale electroanalysis, which has the potential to facilitate advanced research in energy storage.
References
- Güell, A. G., Ebejer, N., Snowden, M. E., Macpherson, J. V., & Unwin, P. R. (2012). Journal of the American Chemical Society, 134(17), 7258-7261.
- Güell, A. G., Cuharuc, A. S., Kim, Y. R., Zhang, G., Tan, S. Y., Ebejer, N., & Unwin, P. R. (2015). ACS nano, 9(4), 3558-3571.
- Gao, R., Edwards, M. A., Qiu, Y., Barman, K., & White, H. S. (2020). Gao, R., Edwards, M. A., Qiu, Y., Barman, K., & White, H. S. (2020). Journal of the American Chemical Society, 142(19), 8890-8896.
- McKelvey, Kim, et al. “Nanopipettes as a tool for single nanoparticle electrochemistry.” Current Opinion in Electrochemistry 6.1 (2017): 4-9
- Bentley, Cameron L., Minkyung Kang, and Patrick R. Unwin. Analytical Chemistry 92 (2020): 11673−11680
- Wang, Yufei, Emma Gordon, and Hang Ren. Analytical Chemistry 92.3 (2020): 2859-2865.
- Shi, W., Goo, D., Jung, G., Pascual, G., Kim, B., & Lee, K. Simultaneous Topographical and Electrochemical Mapping using Scanning Ion Conductance Microscopy–Scanning Electrochemical Microscopy (SICM-SECM).
- Novoselov, K. S.; Geim, A. K.; Morozov, S. V.; Jiang, D.; Zhang, Y.; Dubonos, S. V.; Grigorieva, I. V.; Firsov, A. A. Science 2004, 306, 666−669.
- Bentley, Cameron L., David Perry, and Patrick R. Unwin. Analytical chemistry 90.12 (2018): 7700-7707.
- Ushiki, Tatsuo, et al. Micron 43.12 (2012): 1390-1398.
- Snowden, M. E., A. G. Güell, S. C. S. Lai, K. McKelvey, N. Ebejer, M. A. O’Connell, A. W. Colburn, P. R. Unwin, Anal. Chem., 2012, 84, 2483-2491.
Acknowledgments
Produced from materials originally authored by Jiali Zhang and Byong Kim from Park Systems Inc
This information has been sourced, reviewed and adapted from materials provided by Park Systems.
For more information on this source, please visit Park Systems.