Stirring can allow the dispersion of substances evenly in liquid. Einstein’s tea leaf paradox is a concept that shows how tea leaves can concentrate in a doughnut shape through a secondary flow effect during stirring. In a new study published in Science Advances, Zehui Zhang and colleagues in physics and engineering in China, demonstrated the Einstein’s tea leaf paradox (abbreviated as ETLP) induced concentration in nanofluids.
They accomplished this by simulating the nanoparticle trajectory under stirring to obtain a grayscale analysis of nanofluids under stirring and standing processes. The team applied the localized concentration to achieve ultrafast aggregation of gold nanoparticles to form gold aerogels. They adjusted the gold aerogels from about 10 to 200 nm and developed a constituent of extremely high purity and crystallinity to reveal potential applications in photocatalysis and surface-enhanced Raman scattering.
Einstein’s tea leaf paradox
In 1926, Albert Einstein described a simple experimental observation while stirring tea, where the leaves followed a spiral trajectory towards the center of the cup. Accordingly, the gathering of tea leaves under stirring due to the secondary flow is useful to collect microscale particles in dispersion systems. Since nanoparticles with better stability usually move together with the fluid due to Brownian motion, during Einstein’s tea leaf paradox, the flow velocity paradox induced laminar flows, driving the localized concentration or aggregation of colloidal nanoparticles inside the thin flow.
Materials scientists have focused on metal aerogels such as gold, in catalysis, absorption, and device biocompatibility applications, as well as in electrochemistry. Typically, three main routes can be used to prepare metal aerogels. In this work, Zhang and colleagues showed the localized aggregation of gold nanoparticles and the regulation of the microstructures of gold aerogels. The Einstein’s tea leaf paradox-induced localized aggregation of metal particles pave the way to other types of gels or aerogel production.
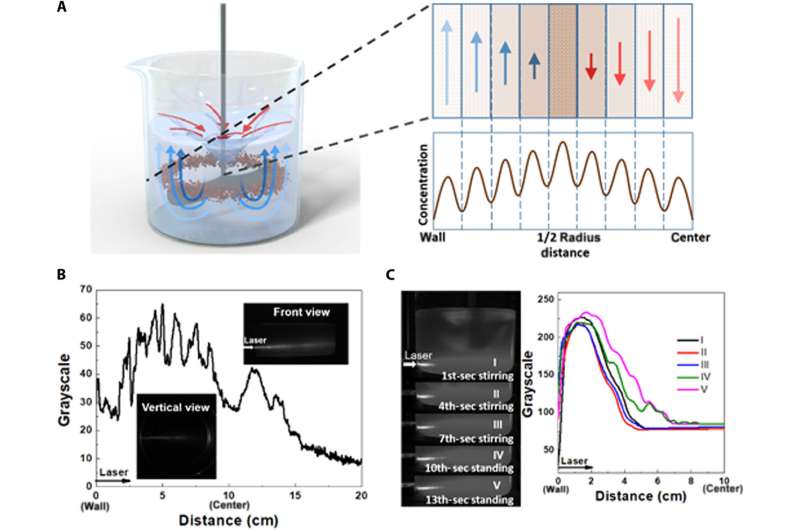
Demonstrating the protocol in the nanofield
The scientists studied the relationship between nanoparticle distribution and flow velocity in nanofluids by using COMSOL Multiphysics software to recreate the movement of nanoparticles in laminar flow under stirring. They monitored the nanoparticle trajectory after stirring for 500 seconds, where the nanoparticles in the middle moved faster with a longer trajectory. The high motion frequency and amplitude of the nanoparticles in the high-velocity regions promoted the encounters of nanoparticles to make them more concentrated or crosslinked.
Based on the outcomes, Zhang and team assumed that the motion of nanoparticles in nanofluids would follow the ETLP (Einstein’s tea leaf paradox) law. To demonstrate the ETLP law at the nanoscale, the team dispersed the 50 nm spherical silicon dioxide nanoparticles in deionized water as a nanofluid. The nanoparticles exhibited macroscopic ETLP with localized concentration effects in nanofluids.
![Assemble-disassemble process in HAuCl4 solution. (A) The color change of HAuCl4 solution when heated and cooled down: HAuCl4 solution heated at 30°, 50°, and 80°C for 1 hour, respectively, and then cooled down to 10°C. (B) Supposed mechanism of Au ion cluster construction: [AuCl4]− may be dechlorinated and cochlorinated to form large Au ion clusters. (C) hν-αhν graph converted from fig. S10A (UV-Vis of HAuCl4 solution was measured from 80°C to room temperature continuously four times). (D) Raman shift of 2.5% HAuCl4 solution during heating and cooling processes. a.u., arbitrary units. (E) FTIR spectra of 10% HAuCl4 solution measured continuously three times from 80°C to room temperature. (F) The whole preparation process. The combination of [AuCl4]− could be used to control the skeleton size of GAs. Credit: Science Advances (2023). DOI: 10.1126/sciadv.adi9108 Investigating the Einstein's tea leaf paradox to study nanofluids](https://scx1.b-cdn.net/csz/news/800a/2023/investigating-the-eins-2.jpg)
Developing gaseous aerogels
The research team prepared a locally aggregated gold gel by reducing gold ion clusters through Einstein’s tea leaf paradox process. They formed chloroauric acid (HAuCl4) solution with the gold clusters and dried the constituents at room temperature or under a heating source of light for transmission electron microscopy observations.
Under light heating, the particles gathered into clusters, which the team further observed with measurements and analysis. These included conductivity and pH value of the gold solution measured during the heating and cooling processes. By regulating the temperature of the precursor solution, the researchers prepared three gold aerogel samples through stirring within 20 minutes. However, without stirring, there was no obvious gel formation in gold solution, even after 24 hours and at 80°C.
Characterization and applications of gold nanoparticles
Zhang and colleagues analyzed the skeleton microstructure of the aerogels by using small angle X-ray scattering, scanning electron microscopy and transmission electron microscopy. The size of gold particles in the aerogel were notably different.
Using X-ray photoelectron spectroscopy, the scientists detected the elemental composition of three samples. Aside from carbon from a source of contamination, they observed only gold in the composition of the aerogels. The preparation process had a significant time-preserving quality, forming gold aerogels with a large range of microstructure sizes and high purity.

Outlook
In this way, Zehui Zhang and team confirmed the Einstein’s teal leaf paradox (ETLP) to be applicable to nanofluids with an unexpectedly localized aggregation effect to form gold aerogels by simply stirring.
The scientists constructed gold ion clusters of different sizes by regulating the temperature of chloroauric acid. They completed the experiments with ETLP-driven aggregation effects and carbon dioxide drying to develop aerogels with varying skeleton sizes, with a capacity for future aerogels to be prepared similarly.


