Understanding the dissolution processes of minerals can provide key insights into geochemical processes. Attempts to explain some of the observations during the dissolution of calcite (CaCO3) have led to the hypothesis that a hydration layer forms, although this has been contested.
Hydration layers are also important as they play a role in a number of processes including adhesion, corrosion and wetting, as well as the folding, stability and recognition of proteins.
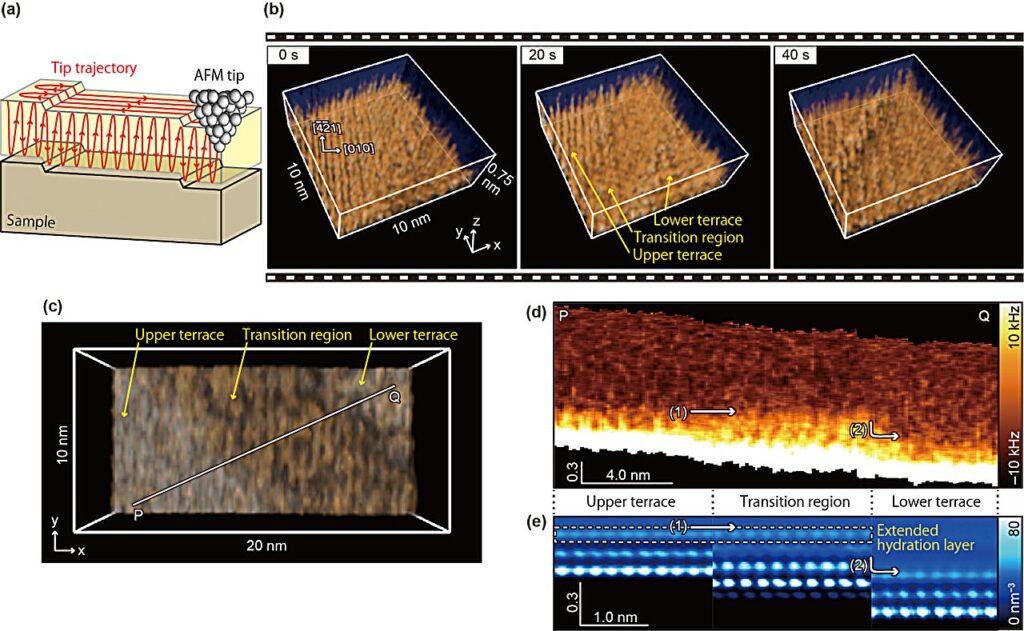
Now researchers led by Kazuki Miyata, Adam S. Foster and Takeshi Fukuma at the Nano Life Science Institute (WPI-NanoLSI) at Kanazawa University in Japan have successfully upgraded their atomic force microscope to retrieve imaging data with the time and spatial resolution needed to obtain 3D structure images that provide direct evidence of a hydration layer forming during the dissolution of calcite.
The research is published in the journal Nano Letters.
The hypothesis of a hydration layer forming during the dissolution of calcite was prompted by simulations of the process, which pointed to the production of a Ca(OH)2 layer across “transition regions” as calcite dissolves.
Despite being unstable in the bulk or on flat terraces, Ca(OH)2 can appropriate some stability from step edge structures, although the mechanism behind this is not well understood.
This could explain the stability of the Ca(OH)2 next to the step edges but since the transition regions observed in experiments span several nanometers, the authors had posited the possibility that the Ca(OH)2 acquires its stability through indirect interactions with the step by means of a hydration structure.
However, as the researchers point out in their report, hydration effects remain “poorly understood” as techniques for imaging changes in solid–liquid interfacial structures are lacking.

Atomic force microscopy (AFM) obtains high resolution images by using a nanoscale cantilever to feel the surface a little like the needle of a record player feels the grooves in vinyl. However, despite a huge step change in the rate of image acquisition that can be achieved with the invention of high-speed (HS) AFM, AFM has still suffered a little from a trade-off between speed and spatial resolution.
Efforts to apply it to the study of dissolution processes are also hampered because the tool is designed to scan the topologies and interactions across 2D surfaces, and dissolution of minerals involves 3D structural changes.
Previous work had expedited the higher resolution “frequency modulated” (FM) AFM so that the image acquisition time was reduced from a minute to just 0.5 s/frame. This upgrade allowed the authors to image the transition region from which they inferred the presence of a hydration layer, but some extrapolation was required to extract 3D structure information from comparison of the 2D-AFM data to 3D simulation, leaving some to doubt the conclusions.
Modifications of AFM to extract 3D force data using AFM have previously been demonstrated, although once again, despite some improvements to speed things up, to about 1 minute/frame the image acquisition time had remained prohibitive for observing dynamic processes.
The authors get around all these drawbacks by combining the HS-FM-AFM with 3D-SFM. This involved increasing the bandwidth of their 3D-SFM while maintaining a force resolution of 10-100nN, fast synchronization of the signals in the lateral scanning and third dimension, and fast recording of the cantilever frequency shifts. With these in place, the researchers were able to capture 3D-SFM images in just 1.6 s/frame. They used the approach to image the dissolution of calcite.
“The HS-3D-SFM images produced in the present work clearly show the 3D distribution predicted by the simulations, thus supporting the existence of an extended hydration layer,” they point out in their report.