In nature and technology, crystallization plays a pivotal role, from forming snowflakes and pharmaceuticals to creating advanced batteries and desalination membranes. Despite its importance, crystallization at the nanoscale is poorly understood, mainly because observing the process directly at this scale is exceptionally challenging. My research overcame this hurdle by employing state-of-the-art computational methods, allowing them to visualize atomic interactions in unprecedented detail.
Published in Chemical Science, my research has uncovered new details about how salt crystals form in tiny nanometer-sized spaces, which could pave the way for advanced materials and improved electrochemical technologies.
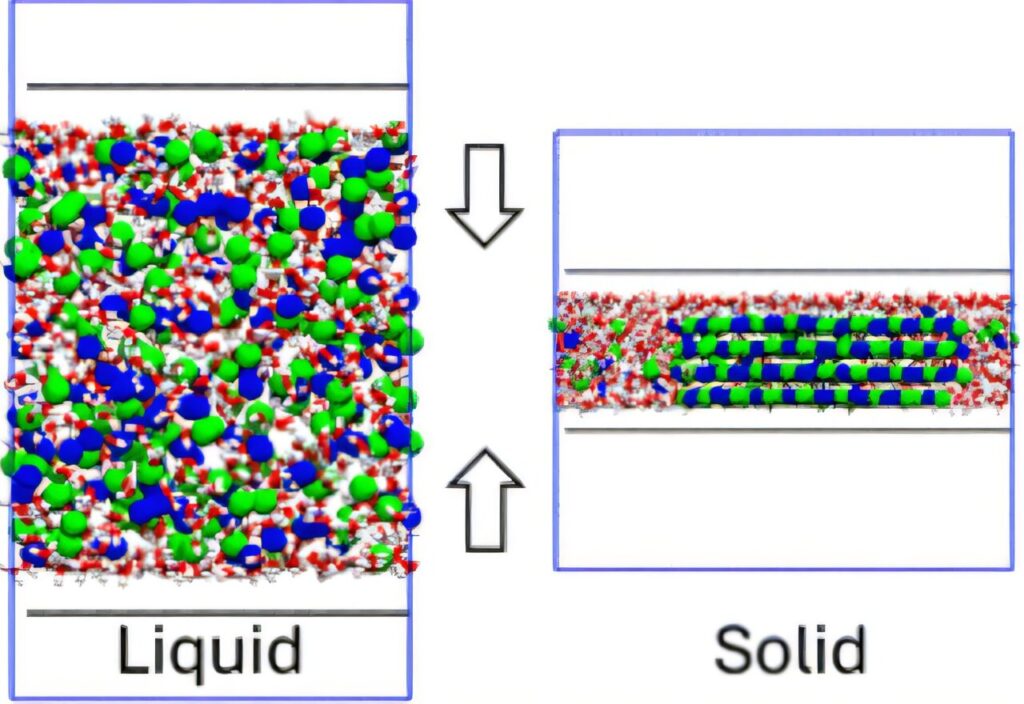
This research used sophisticated molecular dynamics simulations enhanced by cutting-edge machine learning techniques to study how sodium chloride (NaCl), common table salt, crystallizes when confined between two graphene sheets separated by just a few billionths of a meter. These extreme conditions, known as nano-confinement, drastically alter how molecules behave compared to bulk, everyday conditions.
Understanding how crystallization occurs in nano-confined spaces opens the door to precise control over crystal structures and properties. These findings could lead to revolutionary advances in nanotechnology, energy materials, and chemical engineering.
The study revealed several remarkable findings. Most notably, I observed that confinement generally makes the solid salt crystals more stable and significantly increases their melting points, compared to salt in bulk water. This stability depended intricately on the exact spacing between the graphene sheets. At some confinement levels, uncommon crystal structures emerged, including hydrated forms of salt typically stable only at much lower temperatures.
Further analysis using advanced machine learning approaches provided insights into the driving forces behind these unusual crystallization behaviors. The team employed State Predictive Information Bottleneck and Thermodynamically Explainable Representations of AI and other black-box Paradigms techniques to determine critical reaction pathways, revealing the molecular determinants essential for crystal formation under confinement.
The simulations demonstrated that the process of crystallization under these nano-conditions involves carefully orchestrated interactions among ions, water molecules, and their confinement surfaces. Crucially, the team identified that the removal of water molecules directly surrounding ions, particularly chloride ions, played a pivotal role. This water removal, coupled with unique dielectric behaviors under extreme confinement, amplified the Coulomb forces between ions, favoring the formation of solid salt structures.
This fundamental research has far-reaching implications. By precisely understanding the conditions that favor specific crystal structures, scientists can better control processes critical to advanced technological applications. For example, enhanced knowledge of nano-confined crystallization mechanisms could improve the efficiency and stability of electrochemical energy storage devices or lead to better strategies in water purification through advanced desalination membranes.
Additionally, the study introduced a generic computational framework combining enhanced sampling molecular dynamics and machine learning analysis, which could be broadly applied to other complex chemical and physical processes at the nanoscale. This methodology has great potential to uncover new behaviors in confined systems that are central to various areas including energy storage, catalysis, and pharmaceutical manufacturing.
This story is part of Science X Dialog, where researchers can report findings from their published research articles. Visit this page for information about Science X Dialog and how to participate.