By
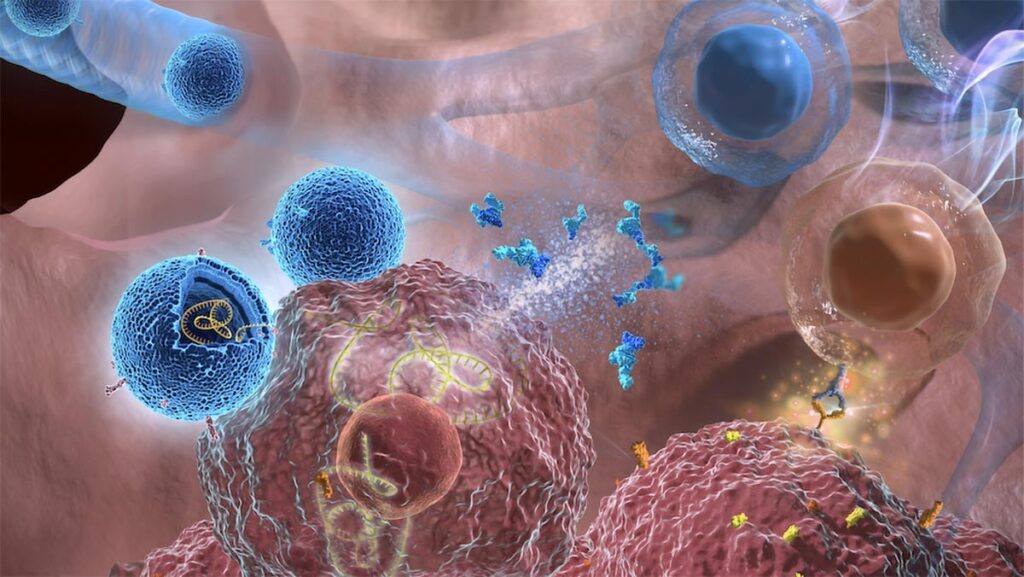
Illustration showing exosomes (blue) deliver IL-12 mRNA to lung cancer cells (brown). Credit: Cheng Lab/Columbia Engineering
Columbia Biomedical Engineer Ke Cheng has developed a technique that uses inhalation of exosomes, or nanobubbles, to directly deliver IL-12 mRNA to the lungs of mice.
Lung cancer is one of the most common cancers and has one of the lowest survival rates in the world. Cytokines, which are small signaling proteins, such as interleukin-12 (IL-12), have demonstrated considerable potential as robust tumor suppressors. However, their applications are limited due to a multitude of severe side effects.
In a paper published recently in Nature Nanotechnology, Biomedical Engineering Professor Ke Cheng and his research group demonstrate that using nanobubbles, called exosomes, through an inhalation treatment method can directly deliver IL-12 messenger RNA (mRNA) to the lungs. mRNAs are the blueprints for producing specific proteins that participate in a variety of cellular functions.
While scientists have previously used liposomes (tiny fat-based particles) or lipid nanoparticles (LNPs) to deliver mRNA, this method has several problems, including a lack of tissue homing, where the particles do not go to the target organs, and concerns about the potential toxicity after long-term exposure. Over the past 15 years, Cheng’s group has been developing exosomes for use as superior drug delivery carriers over liposomes and LNPs in specific indications.
Innovative Delivery Through Inhalation
Up to now, clinicians have only been able to use IL-12 to treat cancer by injecting it directly into the tumor or into the bloodstream. Cheng’s lab found that having the patient — in this case, mice — inhale IL-12 mRNA in exosomes could not only deliver locally concentrated IL-12 into the lungs but also could better fight the cancer with minimal side effects. The inhalation method is more efficient in building higher concentrations of IL-12 right where it is needed than other ways of delivering mRNA such as using liposomes.
“Exosomes are usually injected systemically into the bloodstream,” said Cheng. “In this new study, we show that inhaled exosomes can efficiently reach the lung and deliver an anti-lung cancer cargo, IL-12 mRNA. This is a major step forward in advancing the development of new inhalable drugs to treat lung cancer, which has one of the lowest five-year survival rates in the world.”
Immune System Activation and Tumor Resistance
Inhaling the nanobubbles with the IL-12 blueprint can kickstart the lung immune cells, turning them into powerful defenders equipped to release substances that directly target and destroy tumor cells. In addition, IL-12 helps train these immune cells to “remember” the unique features of tumor cells. As a result, if the tumor tries to attack again, these well-informed immune cells are ready to recognize and eliminate the tumor swiftly.
Additionally, these supercharged immune cells can spread their newfound knowledge to other, untrained immune cells throughout the body, creating an army of defenders. This means that even if tumor cells try to spread beyond their original location, like the lungs, these prepared immune cells can spot and wipe them out, offering a body-wide defense system against cancer. The mice that inhaled this therapy demonstrated lung tumor suppression as well as heightened resistance against tumor rechallenges.
Combining Efficacy With Simplicity
This strategy stands out as a potent IL-12 mRNA delivery system to the lung microenvironment, say the researchers, and combines simplicity with efficacy against primary tumors and metastases. Compared to other nanoparticle controls, exosomes boost IL-12 expression with mitigated toxicity. And patients are likely to be much happier with simply inhaling the therapeutic rather than receiving intratumoral injections.
Future Directions
Cheng’s group is now working with Columbia University Irving Medical Center oncologists to translate their results into the clinic to benefit lung cancer patients.
Reference: “Inhalable extracellular vesicle delivery of IL-12 mRNA to treat lung cancer and promote systemic immunity” by Mengrui Liu, Shiqi Hu, Na Yan, Kristen D. Popowski and Ke Cheng, 11 January 2024, Nature Nanotechnology.
DOI: 10.1038/s41565-023-01580-3