Extensive research has been conducted on gold nanostructures due to their captivating electrical, conductive, and optical characteristics. Recently, these materials have expanded beyond their conventional applications, showcasing intriguing functionalities in the fields of biomedical, diagnostic, and catalytic applications.
- High Technology Electronics: Gold nanowires offer a contemporary alternative to carbon nanotubes in the construction of modern touchscreen displays.
- Catalysis: Platinum and palladium nanoparticles demonstrate significant potential in catalytic applications, reducing the overall metal used by over one thousand times.
- Biomedical: Gold nanoparticles play a crucial role in in-vivo photothermal cancer therapy, and they contribute to the cytosomal uptake of drug-loaded nanoparticles for DNA therapy.
- Biodiagnostics: The development of biosensors and multi-channel lateral flow assays facilitates quantitative multi-analyte detection in biodiagnostics.
Gold Nanorods
Over the last two decades, researchers have employed spherical gold nanoparticles in the study of photothermal cancer therapies.
However, they were not optimized because spherical gold nanoparticles have limited peak absorptions (maximum ~580 nm for 100 nm diameter particles), which fall below the transmission window of 650 – 900 nm for biological entities (skin, tissue and hemoglobin).
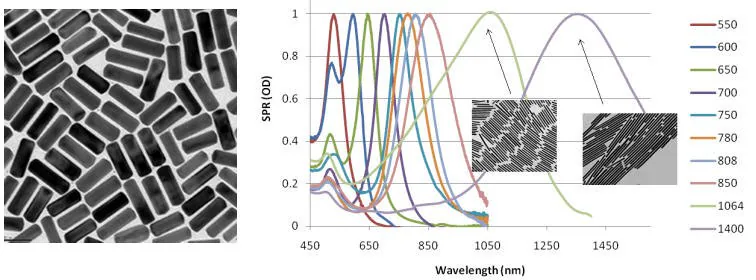
In contrast, gold nanorods exhibit similar behavior but are elongated to optimize their peak absorption and scattering characteristics, enabling tunable absorption from 550 nm to 1400 nm through various manufacturing processes (Figure 1). This tunability allows gold nanorods to scatter wavelengths across the visible and near-IR regions.
The Surface Plasmon Resonance (SPR) value, measuring surface electromagnetic waves along the metal/solvent interface, is highly influenced by materials adsorbed onto the metal surface or metal nanoparticles.
Very precise absorption values for metal nanostructures can be achieved by measuring the SPR values at various wavelengths (Figure 1).
Figure 1. Left: TEM image of typical gold nanorods (10 nm x 40 nm) (Product No. 716820). Right: Gold nanorod peak absorption and scattering (extinction) can be tuned across the visible and near IR spectra shown on a plot of Surface Plasmon Resonance (SPR) to Wavelength. Image Credit: Merck
Gold nanorods are increasingly utilized to augment in-vivo imaging through photoacoustic methods and highly efficient non-linear optical processes, including four-wave mixing.
The Centre for Micro-Photonics at Swinburne University of Technology in Victoria, Australia, leverages these materials as polarizers (Figure 2), substantially enhancing DVD storage capacities by more than 2000 times.
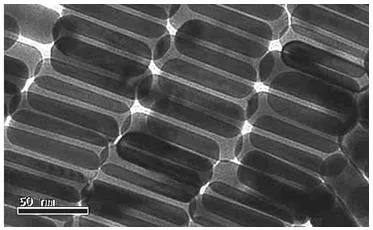
Figure 2. TEM image of gold nanorods used as polarizing materials. Image Credit: Merck
Functionalized Gold Nanorods
Sigma-Aldrich Materials Science provides a range of functionalized gold nanorods featuring diverse functionalities, including methyl (716901), amine (716871), and carboxyl (716898) terminal groups.
The amine and carboxyl terminated nanorods are particularly beneficial for linking various biomolecules such as proteins, antibodies, and even DNA.
In the visible region, these nanorods find application in multi-channel lateral flow assays, while in the near-IR region, they can be intravenously administered, aggregating around tumors. Concentrated around diseased cells, they respond to continuous wave near-infrared lasers, generating heat for photothermal cancer therapy.
Gold nanostructures, serving as both drug delivery vectors and diagnostic tools, can simultaneously target and eliminate cancer cells using pulsed lasers—a merging of diagnostics and therapy called theranostics (Figure 3).
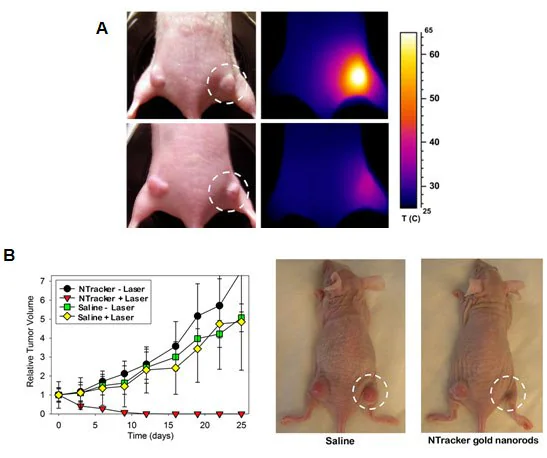
Figure 3. A) Photothermal cancer therapy in mice using gold nanorods and an external diode laser to heat and destroy the cancer cells (courtesy of Nanopartz, Inc.). B) Ablation of cancer cells using pulsed near infrared laser and gold nanorods (courtesy of Nanopartz, Inc.).
Gold Nanowires
Gold nanowires that are 30 nm axially and up to 20 microns in length are finding use as an alternative or complimentary material to carbon nanotubes. They are highly conductive and more transparent, and unlike silver nanowires, they are resistant to corrosion or oxidation.
Nanowires can also replace carbon nanotubes in touchscreen displays and transparent electrodes (Figure 4). Gold nanowires also demonstrate promise as highly sensitive electronic biosensors.
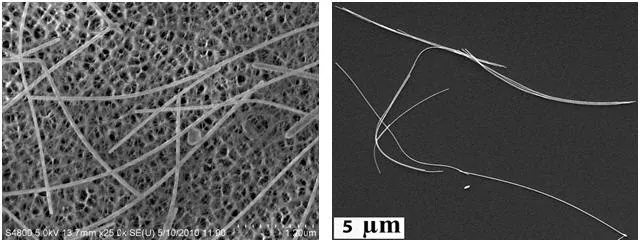
Figure 4. Left: TEM image of gold nanowires (Product Nos. 716944 and 716952) on a carbon nanotube framework. Right: SEM image of gold nanowires that can be used as a carbon nanotube replacement in organic electronics applications. Image Credit: Merck
Microgold
Microgold, spanning hundreds of nanometers in width and up to 1 micron in length, represents the upper limit of particle size classified as a nanoparticle. Its distinct dimensions dictate exceptional absorption and optical properties, rendering it a unique material in this regard.
Notably, Microgold (716960) stands as the first gold particle observable under white light microscopy using conventional optics at single-particle sensitivities (Figure 5).
With a proprietary polymer coating, it is the first particle capable of cellular endocytosis into the cytosome (Figure 5), a crucial aspect in cell therapies aiming to circumvent uptake by cell liposomes.
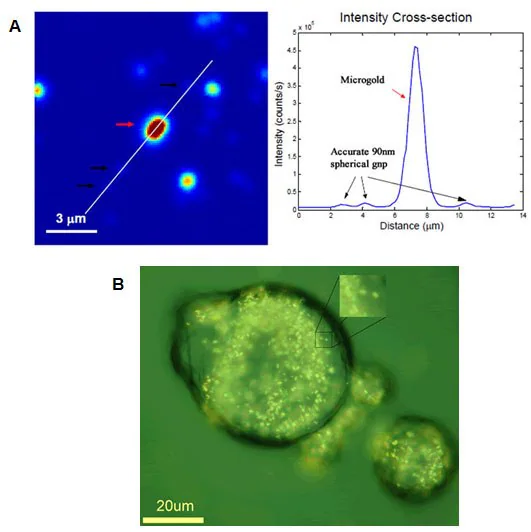
Figure 5. A) Microgold (Product No. 716960) used for single particle measurements in light microscopy (courtesy of Dr. Stephan Link, Rice University). B) Microgold used for cytosome uptake in cancer cell for drug delivery (courtesy of Dr. Eugene Zubarev, Rice University).
Platinum and Palladium Coated Gold Nanostructures
The miniaturization of gold structures to the nanoscale, as discussed above, results in enhanced properties ranging from electrical to biomedical applications. However, at a very basic level, decreasing the size also dramatically increases the surface area, making nanomaterials an interesting candidate for use in catalysis.
Gold nanoparticles can be reliably synthesized to be monodisperse, so they are a good platform to support platinum (716936) or palladium (716928) coatings for catalysis.
When particles are monodisperse, their complete surface area can be utilized, which allows for more efficient catalysis. Both the size decrease and monodispersity create surface areas that are up to ten times greater than conventional platinum and palladium nanoparticles and thousands of times greater than bulk materials (Figure 6).
This increased surface area leads to greener processes by enhancing catalytic efficiency and minimizing waste production.
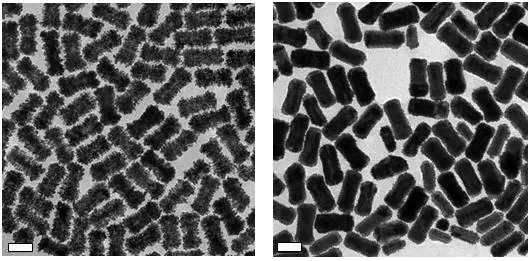
Figure 6. TEM images of Platinum coated gold nanostructures (Left) and Palladium coated gold nanostructures (Right). Scale bars are 50 nm. Image Credit: Merck
Summary
The scope of applications and varieties of gold nanoparticles has broadened substantially beyond their initial use in bulk aggregation responses typical of lateral flow assays, such as those found in pregnancy tests. Their single nanoparticle properties are now being used in biomedical, material, optical, and industrial applications.
These materials are still being actively researched, and applications utilizing their enhanced photothermal capabilities and surface reactivities are only beginning to be realized.
References
- Declerck V, Ribière P, Martinez J, Lamaty F. 2004. Sequentialaza-Baylis?Hillman/Ring Closing Metathesis/Aromatization as a Novel Route for the Synthesis of Substituted Pyrroles. J. Org. Chem.. 69(24):8372-8381. https://doi.org/10.1021/jo048519r
- Schwartz O, Oron D. 2009. Background-Free Third Harmonic Imaging of Gold Nanorods. Nano Lett.. 9(12):4093-4097. https://doi.org/10.1021/nl902305w
- Zijlstra P, Chon JWM, Gu M. 2009. Five-dimensional optical recording mediated by surface plasmons in gold nanorods. Nature. 459(7245):410-413. https://doi.org/10.1038/nature08053
- Venkataramasubramani M, Tang L. 2009. Development of Gold Nanorod Lateral Flow Test for Quantitative Multi-analyte Detection.199-202. https://doi.org/10.1007/978-3-642-01697-4_73
- Fourkal E, Velchev I, Taffo A, Ma C, Khazak V, Skobeleva N. 2009. Photo-Thermal Cancer Therapy Using Gold Nanorods.761-763. https://doi.org/10.1007/978-3-642-03885-3_211
- Lukianova-Hleb EY, Hanna EY, Hafner JH, Lapotko DO. 2010. Tunable plasmonic nanobubbles for cell theranostics. Nanotechnology. 21(8):085102. https://doi.org/10.1088/0957-4484/21/8/085102
- Hu L, Kim HS, Lee J, Peumans P, Cui Y. 2010. Scalable Coating and Properties of Transparent, Flexible, Silver Nanowire Electrodes. ACS Nano. 4(5):2955-2963. https://doi.org/10.1021/nn1005232
- Yogeswaran U, Chen S. A Review on the Electrochemical Sensors and Biosensors Composed of Nanowires as Sensing Material. Sensors. 8(1):290-313. https://doi.org/10.3390/s8010290
- Liu Z, Searson PC. 2006. Single Nanoporous Gold Nanowire Sensors. J. Phys. Chem. B. 110(9):4318-4322. https://doi.org/10.1021/jp056940t
This information has been sourced, reviewed and adapted from materials provided by Merck.
For more information on this source, please visit Merck.