The tumor microenvironment—an ad hoc, messy amalgamation of signaling molecules, immune cells, fibroblasts, blood vessels, and the extracellular matrix—acts like a “powerful security system that protects solid tumors from invaders seeking to destroy them,” says Michael Mitchell, a bioengineer at the University of Pennsylvania working on nanoscale therapeutics aimed at targeting cancers.
“A lot like the Death Star with its surrounding fleet of fighter ships and protective shields, solid tumors can use features like immune cells and vasculature to exert force, acting as a physical barrier to rebel forces (nanoparticles) coming in to deliver the payload that destroys it,” Mitchell says.
Now, researchers in the Mitchell lab have teamed up with Wei Guo’s group in the School of Arts & Sciences at Penn and Drew Weissman of the Perelman School of Medicine to figure out the molecular mechanisms that make tumor microenvironments seemingly impenetrable and found that small extracellular vesicles (sEVs) are secreted by tumor cells and act as a “forcefield,” blocking therapeutics.
Their findings are published in Nature Materials.
“This discovery reveals how tumors create a robust defense system, making it challenging for nanoparticle-based therapies to reach and effectively target cancer cells,” Guo says. “By understanding the cellular mechanisms driving these responses, we can potentially develop strategies to disable this defense, allowing therapeutics to penetrate and attack the tumor more efficiently.”
The research builds on a prior collaboration between Guo and Mitchell’s labs, wherein the teams focused on how tumor-associated immune cells, known as macrophages, contribute to the suppression of anti-tumor immunity by secreting extracellular vesicles.
Wenqun Zhong, a research associate in the Guo lab, says they demonstrated that tumor tissues release a significant amount of sEVs carrying a protein that blocks the activity of cytotoxic T cells, a white blood cell that normally kills cancer cells and other cells that were infected with invaders like viruses or bacteria.
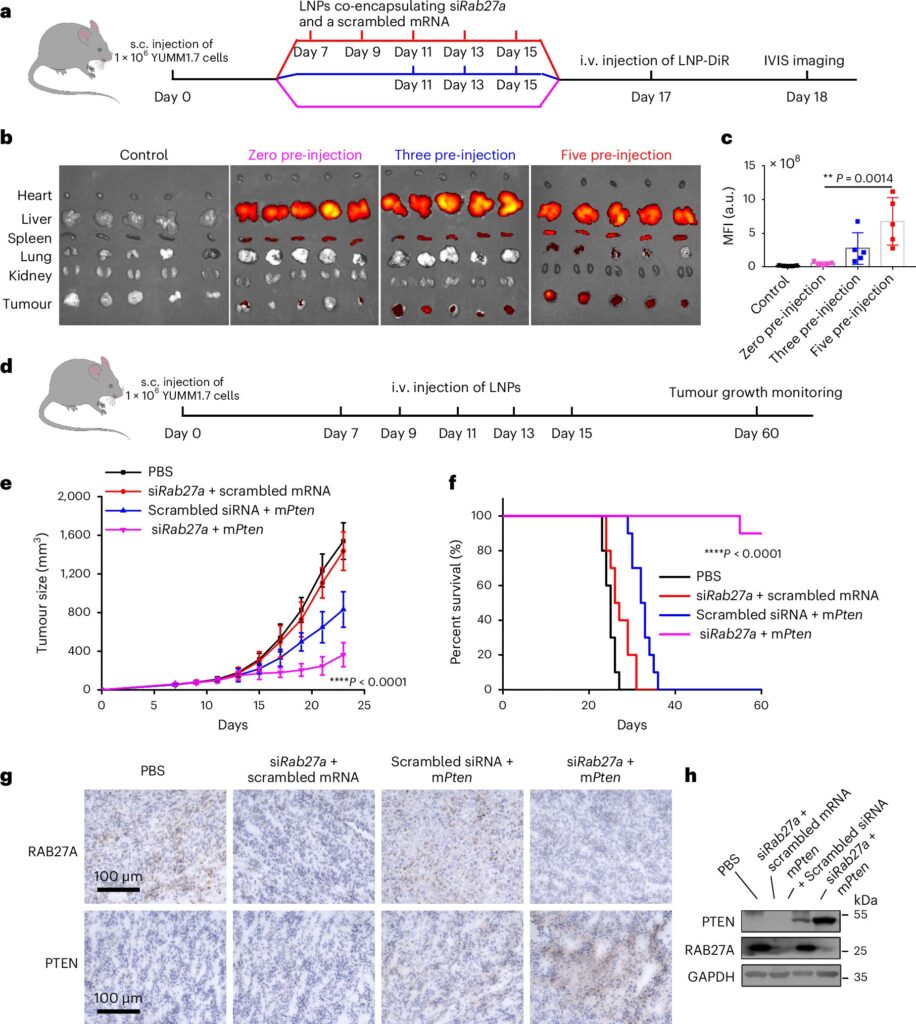
This laid the groundwork for further investigation, leading the researchers to team up again and shift their focus from the role of the tumor cells in a bid to figure out how these sEVs not only suppress immune activity but also block nanoparticles.
The researchers used CRISPR-Cas9, a gene-editing tool, to knock out Rab27a, a gene known to play a major role in sEV secretion, as they “wanted to see if halting the secretion would allow the STING mRNA-loaded lipid nanoparticles to penetrate the tumor tissue more effectively,” says first author Ningqiang Gong, a former postdoctoral researcher in the Mitchell lab.
“But what we found was more than just a reduction in the forcefield effect: The sEVs also acted as a decoy, intercepting the STING mRNA-loaded nanoparticles and diverting them away from the tumor cells like a bouncer escorting an unruly patron at a bar,” Zhong says. “The exosomes come in, pick up the therapeutics, and transport them to the liver where they are degraded by its Kupfer cells.”
In addition to testing STING mRNA-loaded lipid nanoparticles, the team also investigated how other types of nanoparticles and therapeutics interacted with the tumor’s exosome-based defense mechanism.
Gong explains that this included gold nanoparticles, polymeric nanoparticles, and liposomes, and they found that the exosomes secreted by tumor cells acted as a barrier across these different types of nanoparticles, “not just the lipid nanoparticles.”
They even tested therapeutic antibodies that target proteins overexpressed in tumors, such as EGFR (epidermal growth factor receptor), which promotes cell growth, and PD-L1 (programmed death-ligand 1), which helps cancer cells evade the immune system.
The exosomes similarly served as a decoy for these antibodies, diverting them away from their intended targets on tumor cells and reducing their effectiveness.
“The exosomes express the same receptors as the tumor cells,” Gong says. “So, when the antibodies are introduced, the exosomes effectively ‘soak them up,’ diverting them away from the tumor cells.” This diversion meant that fewer antibodies were available to perform their intended function, reducing the overall effectiveness of the therapy.
The team’s findings open new possibilities for improving the delivery of these nanoparticles’ treatments to solid tumors. Moving forward, they plan to explore additional strategies to disrupt this exosome-based defense system and test the approach in different types of tumor types.
“This could potentially lead to more effective treatments for a range of solid tumors, particularly those that are currently resistant to existing therapies,” Mitchell says.