A new study conducted by the Wilhelm Lab at the University of Oklahoma examines a promising development in biomedical nanoengineering. Published in Advanced Materials, the study explores new findings on the transportation of cancer nanomedicines into solid tumors.
A frequent misconception about many malignant solid tumors is that they are comprised only of cancerous cells. However, solid tumors also include healthy cells, such as immune cells and blood vessels. These blood vessels are nutrient transportation highways that tumors need to grow, but they can also be a pathway for medicine delivery, including for cancer nanomedicines.
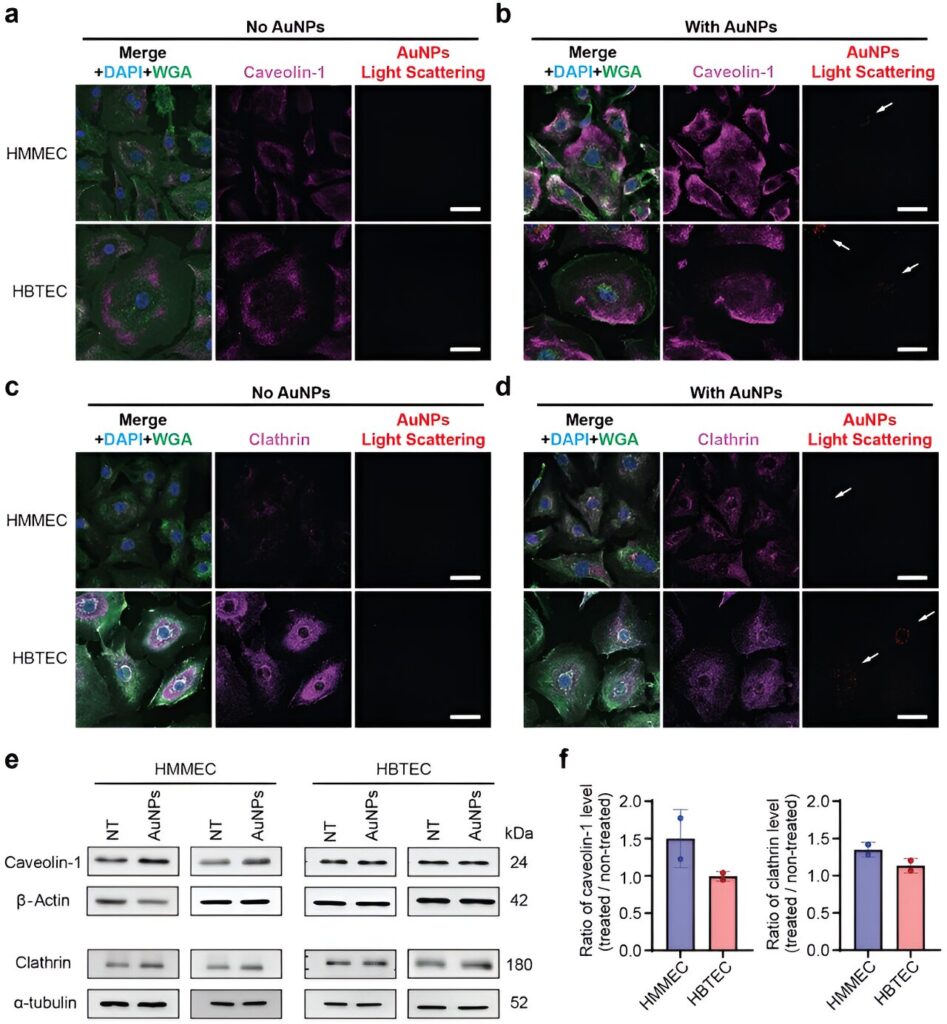
Blood vessels, and the endothelial cells within them, are the transportation method examined in the new study led by Lin Wang, Ph.D., who was a postdoctoral research associate in the Wilhelm Lab while conducting the study and is the first author of the publication. Endothelial cells line blood vessels and manage the exchange between the bloodstream and surrounding tissues. These cells are the first barrier that the nanotechnologies encounter in the process of being transported into tumors.
The researchers found that endothelial cells in breast cancer tumors are two times more likely to interact with medicine-carrying nanoparticles than endothelial cells in healthy breast tissue. Wang said that the tumor endothelial cells have more transport features than the healthy endothelial cells, making them ideal conduits.
“If you know that the same cell type in tumor tissues is two times more likely to interact with your drug carriers than in healthy tissue, then in theory, you should be able to target those cells to get even more nanoparticles delivered into the tumor,” said Stefan Wilhelm, Ph.D., associate professor in the Stephenson School of Biomedical Engineering and corresponding author of the study.
The research was conducted on endothelial cells isolated from breast cancer tissues and isolated from healthy breast tissues. The next steps for the research will involve examining how the nanoparticles react in the context of the whole tissue architecture.
“Cell-culture level experiments are only so good at trying to recapitulate what is happening in the body,” said Wilhelm. “Working with colleagues at OU Health Sciences, we hope to get our hands on not just cells but the entire tumor tissue.”
The research team is working with the Stephenson Cancer Center to set up an ethics protocol allowing the lab to access stored samples of cancer tissue rather than just isolated cells. The Wilhelm Lab is focused on studying nanomedicine and using nanoparticles for drug delivery and diagnostics. In particular, the team is interested in studying the delivery of drugs into solid tumor tissues.
From an engineering perspective, a unique advantage of using nanoparticles for drug delivery is that they are small and flexible enough to be designed as direct delivery vehicles. In a laboratory setting, the nanoparticles are often designed as tiny spheres and loaded with the necessary drugs. Then, in clinics, they are often administered intravenously to patients. These drugs circulate through the bloodstream, and some of them enter the tumor.
There are challenges associated with this type of medicine transportation. One is that these nanoparticles circulate throughout the body, and consequently, they accumulate in other organs—called off-target organs—such as the liver, spleen and kidneys. Since these organs filter blood, they remove the nanoparticles, which are often considered foreign objects by the body.
The field of nanomedicine has been around for more than 40 years, and there are tens of thousands of publications on using nanoparticles to treat cancers at the preclinical stage. But there is a disconnect between the number of preclinical publications and the number of FDA-approved formulations of nanoparticles that are actually used in clinics.
Of those approved formulations, a fraction are used for solid tumors, and most treat liquid tumors, such as leukemia. Wilhelm speculates that this is partially because there is a lack of full understanding of how the nanoparticle delivery process works.
“And if you don’t understand something fully, it’s hard to develop solutions to those problems,” said Wilhelm.
“Researchers have started to go back to the fundamentals of nanomedicine development to understand the translation from the pre-clinical to the clinical space. Our lab wants to focus on these fundamentals to better understand the field and the delivery mechanisms specifically. If we understand these fundamentals, we can contribute even more to the field,” said Wang.
According to Wilhelm, the next big question is this: now that the lab has quantified and shown that endothelial cells are more likely to interact with and transport these nanomedicines, how can that transportation be made more efficient and specific to advance clinical cancer treatments? As these questions are answered, the opportunities for future advances in cancer health care will grow.
“We are just scratching the surface by using breast cancer as our model cancer system, but our findings may be relevant for other types of solid tumors as well,” said Wilhelm.