Researchers at the University of Kentucky have a better understanding of the regulation of extracellular vesicles by oxidative stress and how these vesicles spread oxidative stress and may damage neurons. Extracellular vesicles are nanoparticles released by all cell types that help transport information between cells.
The study titled “Ceramide-mediated orchestration of oxidative stress response through filopodia-derived small extracellular vesicles” was recently published in the Journal of Extracellular Vesicles.
“This study lays the groundwork to learn what’s happening during the exchange of extracellular vesicles between different cell types to then work toward understanding the significance of these processes in neurodegenerative diseases like Alzheimer’s disease,” said Erhard Bieberich, Ph.D., a professor in the Department of Physiology in the UK College of Medicine. Bieberich is the principal investigator.
Bieberich’s research team focused on oxidative stress—an excess of oxygen radicals in the body. This excess results in damage to cells and tissues. Other studies have shown this plays a role in many chronic and degenerative conditions.
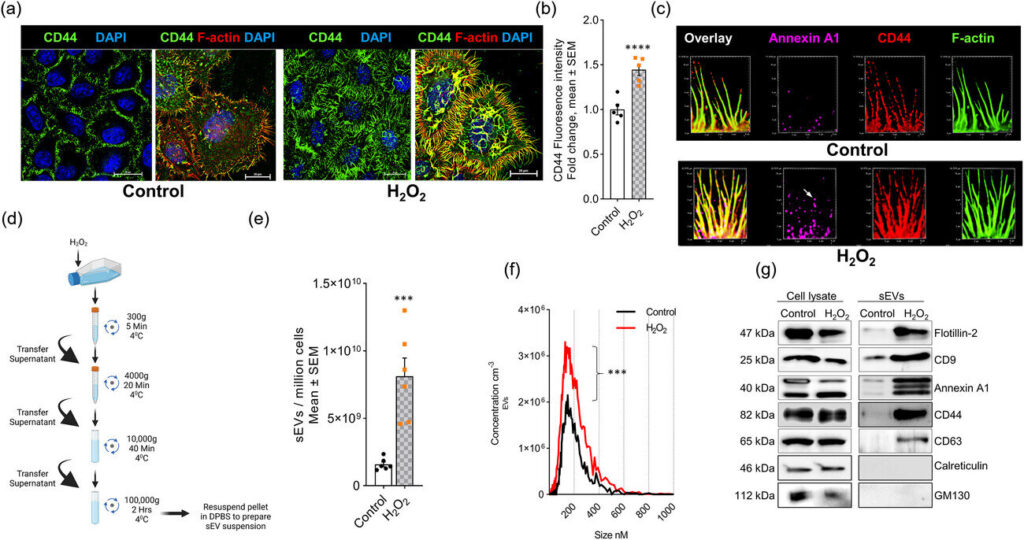
Researchers studied HeLa cells, which are so-called “immortal” cells for their unique ability to continuously grow and divide in the lab. When exposed to hydrogen peroxide, the cells formed filopodia (finger-like projections of cell membranes) and secreted extracellular vesicles (tiny structures released from cell membranes).
“In our study, we investigated the function of ceramide, which is a type of fatty compound, in filopodia formation and extracellular vesicle secretion, both of which are induced by oxidative stress,” said Bieberich.
“Using a novel metabolic labeling technique, we found that those two processes are controlled by two enzymes that generate ceramide at the plasma membrane,” said Zainuddin Quadri, the first author of the study and a senior research associate in the Department of Physiology. “This leads to the release of ceramide-rich small extracellular vesicles from filopodia, which then target mitochondria and cause cell death.”
“Previous research focused on oxidative stress caused by the accumulation of amyloid in plaques or other proteins in neurons,” said Bieberich.
“We discovered a novel mechanism by which oxidative stress can be spread through extracellular vesicles, which may propagate cell death even if neurons themselves are not directly exposed to harmful proteins such as amyloid.”
The two researchers said the induction of cell death was found particularly in neuronal cells, but plan to further study the process in different types of cells.
Their findings suggest that targeting the interaction between the two enzymes could be a potential therapeutic target to prevent oxidative stress-induced cell death.
This research was made possible by the collaboration with two lipid biologists, Stefanka Spassieva, Ph.D., and Mariana Nikolova-Karakashian, Ph.D., in the Department of Physiology. They are also co-authors on the publication.