The skin serves as the body’s primary defense against harmful microorganisms, toxins, and physical damage. However, severe injuries from burns, trauma, surgery, or conditions like diabetes can compromise its ability to heal naturally, necessitating advanced wound care solutions. While minor wounds can heal with conventional care, severe injuries demand innovative materials and techniques to ensure rapid and complication-free healing.
To address this, a team of researchers led by Dr. Saravana Kumar Jaganathan has introduced nanofiber wound patches that combine polyurethane (PU) with magnesium chloride (MgCl2). These advanced patches, developed using electrospinning technology, exhibit enhanced strength, superior blood compatibility, and effective antimicrobial properties. Their findings, published in the International Journal of Nanomedicine, on November 1, 2024, mark a significant advancement in wound care.
“The efficacy of wound management heavily relies on the selection of optimal wound dressings. Our research explores how combining polyurethane and magnesium chloride can address this challenge effectively,” states Dr. Jaganathan.
PU has long been recognized as a versatile material in medical applications due to its flexibility, durability, and biocompatibility. However, Dr. Jaganathan and his team saw the potential for improvement. As he explains, “Wound management is a critical issue. Polymeric wound dressings have garnered wide attention for their ability to accelerate healing, and our study builds on this foundation.”
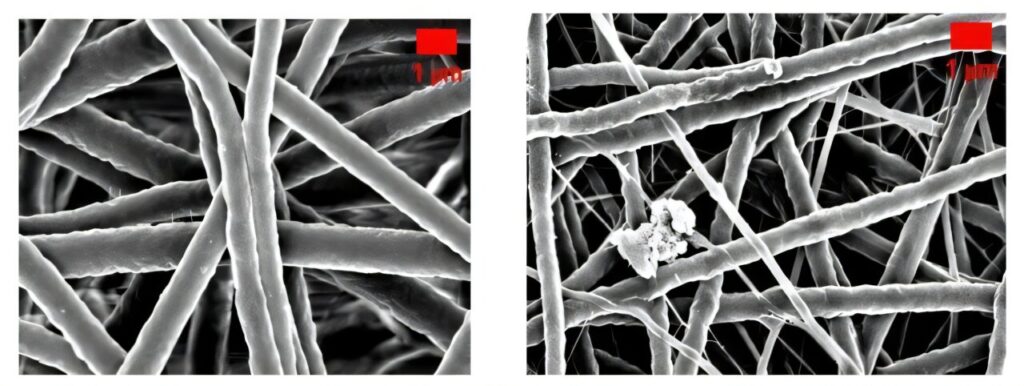
The team utilized electrospinning—a technique that produces nanofibers mimicking the structure of natural tissue to create these innovative patches. This unique structure supports cell attachment and growth while maintaining controlled porosity, which is essential for optimal wound healing. The inclusion of MgCl2 further amplified the patch’s effectiveness, significantly enhancing its mechanical and biological properties.
One standout feature of these patches is their remarkable mechanical strength. Laboratory tests revealed that magnesium-infused patches were almost twice as strong as traditional PU patches, ensuring durability and reliability during clinical use. This makes them particularly suited for handling the demands of severe wound care.
Additionally, the patches demonstrated excellent blood compatibility. Coagulation tests, such as activated partial thromboplastin time and prothrombin time, indicated that MgCl2 delayed clotting time, reducing the risk of adverse reactions and ensuring safer interactions with the body’s healing processes.
Infection control is another critical aspect of effective wound care. The researchers tested the antimicrobial properties of their patches against two common wound-infecting bacteria, Staphylococcus aureus and Escherichia coli.
The results were impressive: the MgCl2-enhanced patches successfully inhibited bacterial growth, while traditional PU patches showed no such activity. This antimicrobial capability significantly reduces the risk of infections, a common and serious complication in wound management.
The team also evaluated the patch’s impact on skin cells, particularly fibroblasts, which are vital for tissue regeneration. Cell viability tests demonstrated that magnesium-infused patches supported fibroblast growth more effectively than conventional PU patches. This non-toxic nature ensures that the patch promotes faster and more efficient healing without compromising safety.
Despite these promising results, the researchers acknowledge that additional studies are needed to validate the patch’s effectiveness in real-world clinical scenarios. In vivo testing will be essential to assess the performance under practical conditions.
This research paves the way for a new era in wound care, promising improved outcomes and enhanced quality of life for those in need of effective wound management solutions.