Like loading dock managers at a shipping warehouse, nuclear pore complexes act as gatekeepers to the headquarters of the cell, controlling traffic out of the nucleus.
A North Carolina State University researcher was part of a new study, published inNano Letters, that revealed a method to better understand what’s going on in images of these tiny pores—a finding they hope scientists can build on to both study nuclear pore complexes, and understand their role in cell development and disease.
Specifically, researchers came up with a method of using machine learning to help differentiate nuclear pore complexes in images of cells. The Abstract spoke to Yang Zhang, assistant professor of textile chemistry, engineering and science at NC State, about the study.
The Abstract: What are nuclear pore complexes?
Yang Zhang: They are nano-sized channels, equipped with proteins, in the membrane of the nucleus. They’re used to transport biomolecules, such as DNA, proteins or other molecules, from the nucleus to the cytoplasm of the cell. They are gatekeepers for many cell activities, such as transcription, which is one of the first steps in turning DNA into proteins.
TA: Why are you interested in nano-sized channels on the nuclear membrane?
Zhang: Traffic through these nuclear pores could control disease pathways. If we are able to program trafficking activities between the nucleus and cytoplasm, we might be able to rewire them to treat diseases, like cancer. We are studying fundamental biological processes using super resolution imaging.
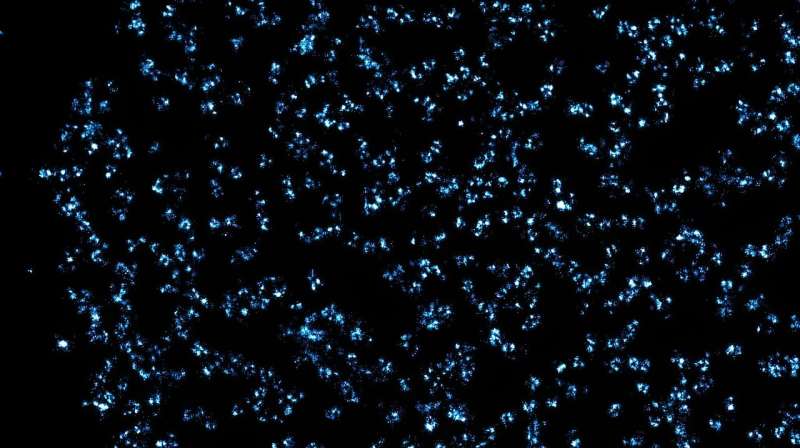
TA: How are you able to take pictures of these tiny nuclear pore complexes?
Zhang: Because these pores are so tiny, they must be imaged using a super-resolution fluorescence microscopy—a Nobel prize-winning technique. More importantly, in order to study these nuclear pore complexes, you can’t just take a high-resolution picture and be done. We developed an approach that allowed us to understand what is happening to the nuclear pore complexes that we see in imaging.
To do that, we first labeled the complexes using fluorescent dyes to be detectable in the super-resolution fluorescence microscope, and then developed a computer simulation of the complex that we compared to the real image.
We compared the simulated image to the real image to know how well we were able to capture the information, and, using machine-learning based imaging segmentation, we were able to better understand the compositions of the complexes. Now, we can build on this to better understand other images of nuclear pore complexes under different conditions.
Zhang was corresponding of the study in Nano Letters.