In antibacterial photodynamic therapy, irradiation is used to produce reactive oxygen species that kill off bacteria. Because it requires external light and oxygen, this method is only suitable for surface infections.
In the journal Angewandte Chemie, a Chinese research team has now introduced a molecular “singlet oxygen battery” that can be “charged” with reactive oxygen, which it then releases in deep tissue layers to target methicillin-resistant staphylococcus.
Antibiotic-resistant bacteria are on the rise. Though often harmless to healthy people, dreaded multidrug-resistant “hospital pathogens” such as methicillin-resistant Staphylococcus aureus (MRSA) use injuries or fresh surgical wounds to gain entry to the body. They also infect immunocompromised patients. Because antibiotics are not effective, there is sometimes no remedy.
One highly promising alternative is antibacterial photodynamic therapy, which is already widely used in dentistry. In this technique, a light-activated substance (photosensitizer) is irradiated, triggering a photodynamic reaction that produces singlet oxygen (1O2), an excited form of oxygen.
Unlike antibiotics, this substance simultaneously attacks multiple biomolecular sites on the bacteria. It is easy to use, safe, painless, and generally free of side effects. Unfortunately, it has only been useful for surface infections because the necessary light only penetrates a few millimeters into the tissue. Additionally deeper tissue layers also do not have enough oxygen for effective treatment.
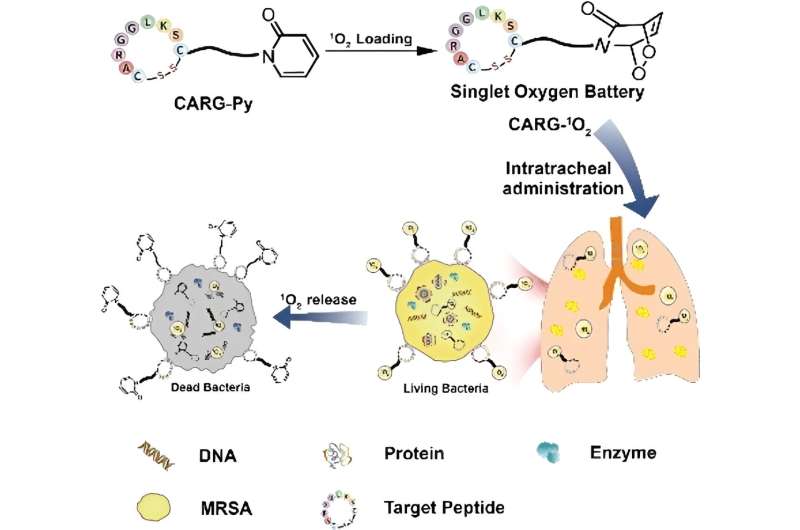
A team led by Bingran Yu and Fu-Jian Xu at Beijing University of Chemical Technology has now developed a new approach to photodynamic therapy: a “singlet oxygen battery” that can be used to fight deep bacterial infections because it requires neither light nor external oxygen.
The conversion of oxygen into reactive singlet oxygen through irradiation in the presence of a molecule that captures light (photosensitizer) happens first. The “battery” is “charged” with the singlet oxygen. This “battery” consists of a special nitrogen-containing, six-membered ring of carbon atoms (pyridone) that tightly binds the singlet oxygen.
The reactive oxygen molecule bridges two opposite vertices of the ring (endoperoxide). A peptide bound to the ring specifically “recognizes” MRSA bacteria, so the molecular batteries accumulate around and in the bacteria and continuously release their singlet oxygen.
The bacteria are thus simultaneously attacked at many different locations, including their membrane, DNA, enzymes, and other proteins. This makes the development of resistance virtually impossible. When administered to mice through nebulization, the singlet oxygen battery was shown to be very effective in treating pulmonary infections caused by MRSA. Systemic side effects were not observed.