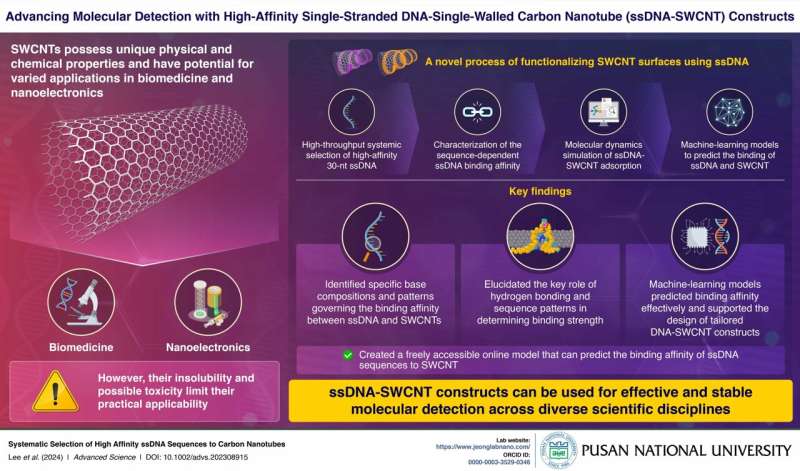
Single-walled carbon nanotubes (SWCNTs) have emerged as promising candidates for applications in biotechnology and nanoelectronics due to their exceptional physical and chemical properties. Despite their potential, challenges like insolubility and toxicity have hindered their widespread use. Prior studies have investigated diverse strategies to functionalize and modify the surfaces of SWCNTs to overcome these challenges.
In a new study, researchers from the Pusan National University led by Professor Sanghwa Jeong, Assistant Professor in the School of Biomedical Convergence Engineering, have attempted to fill this gap. This study has gone beyond conventional techniques by employing high-throughput screening methods to elucidate the relationship between DNA sequences and their binding affinity to carbon nanotubes. It focused on optimizing the binding affinity and stability of these constructs through advanced sequence design and molecular dynamics simulations.
This study is published in Advanced Science.
Discussing the background of their study, Dr. Jeong explains, “Researchers have been exploring various strategies to engineer SWCNT surfaces to overcome the challenges of limited applications owing to insolubility and potential toxicity. One promising approach is the use of single-stranded DNA (ssDNA) as a wrapping surfactant for SWCNTs.”
The researchers employed a rigorous methodology to ensure precise characterization and optimization of single-stranded DNA (ssDNA)-SWCNT complexes. Initially, a diverse random 30-nucleotide (nt) ssDNA library underwent iterative rounds of screening to identify high-affinity sequences.
Computational modeling, particularly molecular dynamics simulations, provided insights into the structural dynamics of the SWCNT constructs. Furthermore, the researchers used several machine-learning models to understand the pattern of sequences that affect binding affinity. They have successfully created a freely accessible online service that predicts the binding affinity of ssDNA sequences to SWCNTs. These integrated approaches not only validated the experimental findings but also guided the design of high-performance ssDNA-SWCNT constructs.
The findings revealed significant advancements in the stability and functionality of ssDNA-SWCNT complexes. High-affinity 30-nt ssDNA sequences, rich in adenine and cytosine, exhibited superior binding strength, validated through surfactant displacement experiments.
Molecular dynamics simulations highlighted the formation of stable intramolecular hydrogen bonds near the SWCNT surface, underscoring their enhanced structural integrity. The machine-learning models effectively predicted the binding affinities of ssDNA sequences, further supporting the design of the tailored ssDNA-SWCNT constructs.
Moreover, the study demonstrated notable improvements in the resistance of these complexes to enzymatic degradation compared to free ssDNA, making them highly suitable for long-term biological applications.
In conclusion, the development of high-affinity ssDNA-SWCNT constructs marks a significant advancement in nanobiotechnology. The exceptional characteristics of ssDNA-SWCNTs make them ideal candidates for cell or tissue-specific drug delivery systems as well as the development of high-performance nano-electronic devices.
Dr. Jeong concludes, “Our study not only makes a substantial contribution to our understanding of the interplay between ssDNA and SWCNTs but also offers practical avenues for harnessing these interactions in a wide range of advanced technologies. In the future, developing nanomaterials and devices with enhanced stability will show promise in driving innovation in nanoelectronics and biotechnology.”

