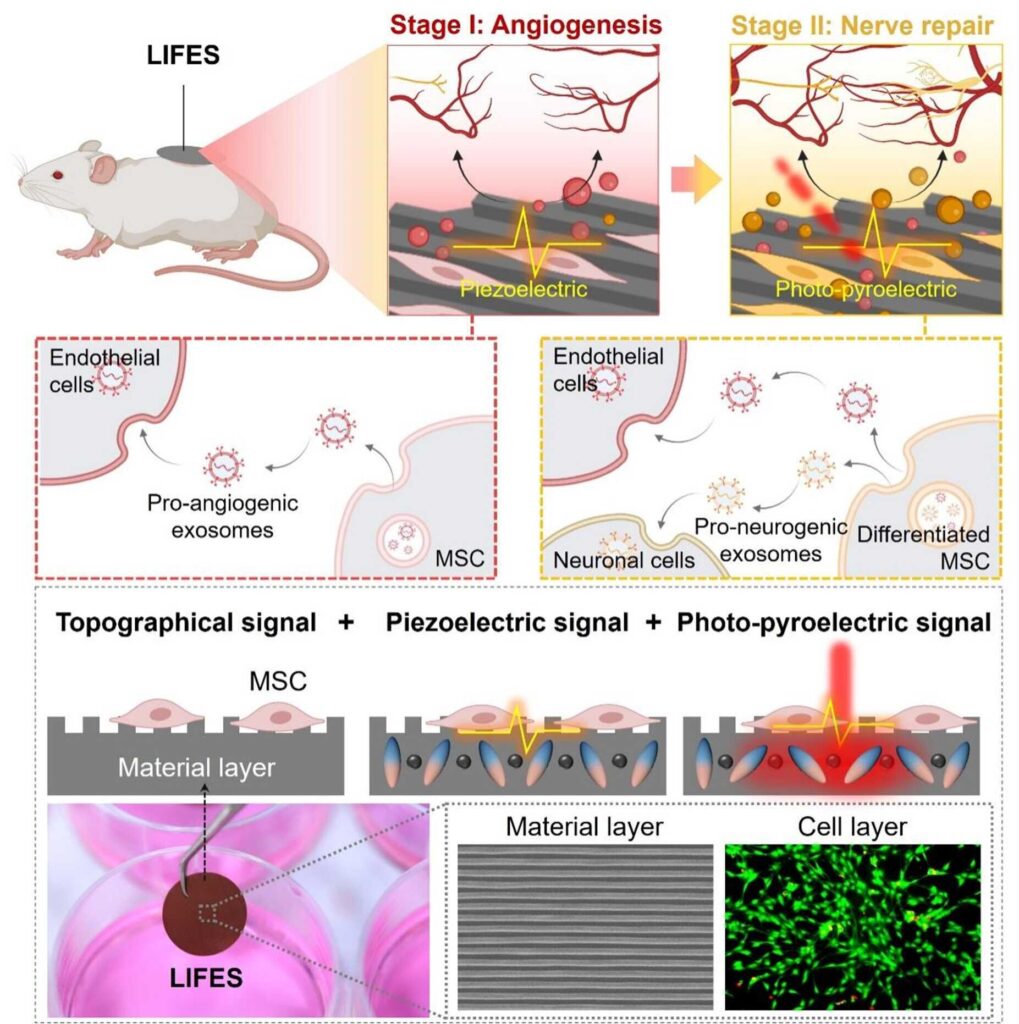
A research team led by Dr. Du Xuemin from the Shenzhen Institutes of Advanced Technology (SIAT) of the Chinese Academy of Sciences has reported a living interface with unique functionalities of durable secretion of bioactive exosomes with tunable contents and miRNA cargoes, effectively promoting neurovascular remodeling.
The study was published in Matter on Nov. 21.
Neurovascular remodeling is crucial for restoring normal functions of regenerated tissues or engineered organs, which requires multi-target and phase-specific paracrine regulation. However, existing strategies still cannot mimic such dynamic and complicated paracrine regulation effects in the native physiological processes, hindering synergistic neurovascular remodeling.
Exosomes, as key entities in the native paracrine process, show great promise for neurovascular remodeling yet still face challenges. Direct exosome administration is limited by its short lifetime (24–48 hours). In addition, exosome delivery systems struggle with preserving bioactivity and maintaining adaptable miRNA cargoes throughout the entire release period, limiting their effectiveness at different stages of neurovascular remodeling.
The proposed living interface in this study for fine-tuned exosome secretion (LIFES) consists of two core elements, a poly(vinylidene fluoride-co-trifluoro ethylene)-based intelligent material layer with rationally designed topographical structures and superior electric properties for cell modulation, and a living cell layer with rat bone marrow-derived mesenchymal stem cells (MSCs) for efficient biogenesis of exosomes.
Through the synergistic interactions between the two core elements, LIFES can secrete bioactive exosomes in sustained (~192 hours) and phase-specific manners, with tunable contents (~8-fold increases) and programmable miRNA cargoes (initially pro-angiogenic and later pro-neurogenic).
“The phase-specific exosome secretion of LIFES meets physiological requirements, which aligns with the native multi-target and multi-stage paracrine regulation effects observed in physiological neurovascular remodeling processes,” said Dr. Du.
By mimicking the natural paracrine regulation effects within the native physiological processes of neurovascular remodeling, LIFES effectively promotes the reconstituting of vascular neural networks, even in challenging diabetic wound models.
The study will open new avenues for next-generation intelligent materials, revolutionizing biomedical devices, regenerative medicine, and brain-machine interfaces.