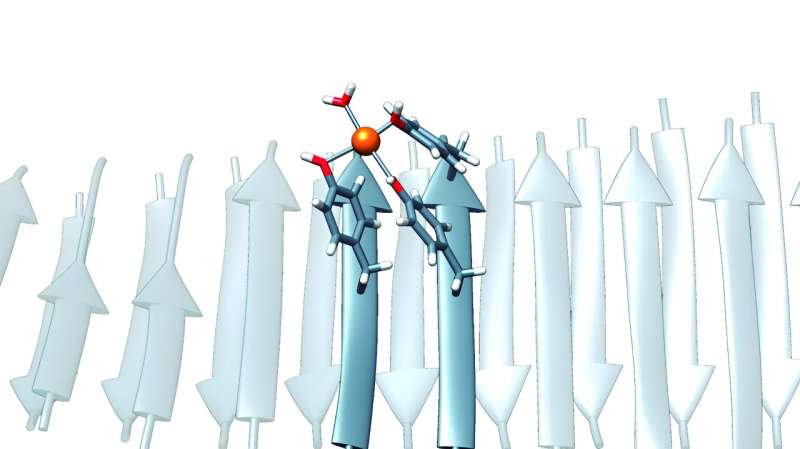
Autonomous University of Barcelona (UAB) researchers have developed minimal nanozymes with the capacity of capturing carbon dioxide (CO2) emitted in industrial processes—and applicable to other environmental remediation processes—based on artificial molecular structures formed by the peptides of only seven amino acids.
These new molecules could also act as metalloenzymes, and this opens up new possibilities in biotechnology research. The study also provides a new contribution to the origin of catalytic activity at the beginning of life.
The research, with Salvador Ventura as coordinator and Susanna Navarro as first author, was recently published in ACS Nano. Both are researchers at the Institute of Biotechnology and Biomedicine and at the UAB Department of Biochemistry and Molecular Biology, and have worked together in the study with researchers from the UAB Department of Chemistry and the Research Center bioGUNE.
In 2018, UAB researchers managed to create very short molecules capable of self-assembly, inspired by the natural ability to self-assemble of amyloid fibrils, and based on a specific sequencing of prion proteins. These artificial amyloids have catalytic activities, with advantages such as modularity, flexibility, stability and reutilisation when compared to natural enzymes.
Now, researchers have discovered their capacity to bind to metal ions effectively and act as metal and metalloenzyme-storage elements.
“These peptides were particular, since they did not contain the typical amino acids, such as histidine, which is often considered essential for the coordination of metal ions in enzymes, and which were thought to be essential for catalytic activity. In contrast, they were enriched with residues from tyrosine, an element which although less known in this context, can also have the unique capacity of binding to metal ions if it finds itself in the correct structural context. Tyrosine’s ability to do so is what we used to create our nanozymes,” Ventura said.
The results can be applied to several areas. First, nanozymes are stable and can be used for environmental remediation, in wastewater treatment processes or contaminated soils, given their notable capacity for sequestrating metal ions.
Second, they can function as metalloenzymes, capable of catalyzing reactions in conditions in which current enzymes, much less stable, would be incapable of acting. This opens up new possibilities in research into biotechnology, such as in catalyzing reactions in extreme temperatures and pH values.
Based on the nanozymes they designed, researchers are convinced that they have successfully developed a minimalistic variant of a carbonic anhydrase enzyme capable of efficiently storing CO2 emitted by greenhouse gases, and at a much lower production cost than natural enzymes.
New perspective on ancestral enzymes
To obtain these new nanozymes, researchers formed the hypothesis that catalytic activity at the origin of life could have emerged as the result of the self-assembling of short, low-complexity peptides into structures similar to amyloids which acted as the primal ancestral enzymes.
“Showing that these molecules have catalytic action without the need of conventional histidine-based coordination represents a significant change in how we understand the origin of catalytic activity at the start of life. We now know that this activity can be achieved if the ancestral peptides contain tyrosine. Therefore, we suggest that it is highly probable that the ancestral enzymes based on amyloids also used this second amino acid in their chemical reactions,” Ventura concludes.
In the study, researchers combined experiments and simulations by using a variety of techniques such as spectrophotometry, flourescence, electron microscopy, electron diffraction, and advanced computational modeling.