Recurring influenza epidemics, such as the one during World War I, the Middle East respiratory syndrome coronavirus (MERS-CoV) outbreak in the 2010s, and the COVID-19 pandemic in recent years have made it evident that contagious viral respiratory diseases often make an appearance in the timeline of human history.
Denser populations, close contact during transportation, and improvements in connectivity have significantly increased the rate of spread of such viral infections.
To minimize viral transmission and mass infection, rapid diagnostic tests that can detect and identify viruses are essential for effective isolation and treatment of infected patients. In recent years, fluorescence-based lateral flow immunoassay (LFI) has gained popularity as a diagnostic tool for viral detection.
It is a rapid virus detection platform which uses molecules that glow under special lighting conditions in the presence of a viral load. However, the performance of this detection platform is limited due to several issues related to detection sensitivity.
In a recent study, a team of researchers led by Professor Min-Gon Kim from the Department of Chemistry at the Gwangju Institute of Science and Technology have now demonstrated that these fluorescence-based LFIs, when enhanced by gold nanorod (GNR)-based probes, could accurately and rapidly detect an influenza virus protein, without the need for complex diagnostic laboratory equipment.
Their work was made available in ACS Nano.
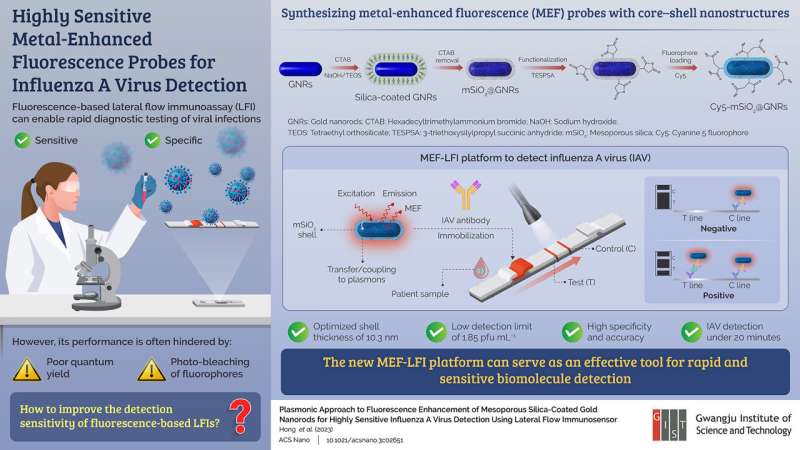
The team developed Cy5-mSiO2@GNR probes with core–shell nanostructures for the LFI platform. These probes consist of a GNR core, a mesoporous silica shell (mSiO2), and the fluorescent molecule cyanine 5 (Cy5). This new biosensing system bypasses common problems associated with fluorescence-based LFI, such as photobleaching of fluorophores and low quantum yields, by leveraging metal-enhanced fluorescence (MEF).
“The platform developed by us uses a phenomenon where light-matter interactions in the vicinity of metal nanoparticles give rise to a plasmonic effect, producing a strong fluorescence. The key factors that dictate this effect are the distance and spectral overlap of the metal and fluorophore in the MEF system,” explains Prof. Kim.
The team then subjected the Cy5-mSiO2@GNR probes to a series of theoretical and experimental tests to investigate the dependence of fluorescence behavior on the distance between the GNR and Cy5 by adjusting the thickness of the mSiO2 shell. They found that a thickness of 10.3 nm was optimal for the shell and accordingly set the morphology condition of the MEF system to achieve an enhanced fluorescence effect.
Furthermore, they demonstrated the applicability of optimized MEF probes by incorporating it onto a LFI platform for the detection of influenza A virus (IAV). Due to the improved fluorescence, the MEF-LFI system was able to detect IAV even at very low concentrations of 1.85 pfu mL-1 within 20 minutes.
It also showed high specificity towards IAV even in the presence of other viruses, such as MERS-CoV and the COVID-19 virus. Furthermore, this biosensing system was able to identify IAV from clinical patient samples with a remarkable accuracy of more than 99%.
Emphasizing the future potential of this platform, Prof. Kim adds, “The findings of this research can not only transform rapid testing in health care, but its scope can be also extended to other forms of biomolecule diagnostics, with the ultimate goal of improving people’s quality of life.”
The new Cy5-mSiO2@GNR-based LFI platform can indeed be a powerful point-of-care diagnostic tool for early detection and screening of IAV and other viruses, even under emergency conditions.