Ethanol fuel cells are regarded as promising sources of green electricity. However, expensive platinum catalysts are used in their production. Research on laser melting of suspensions carried out at the Institute of Nuclear Physics of the Polish Academy of Sciences in Cracow, has led researchers to materials that catalyze ethanol with a similar—and potentially even greater—efficiency to platinum, yet are made of an element that is many times cheaper than platinum.
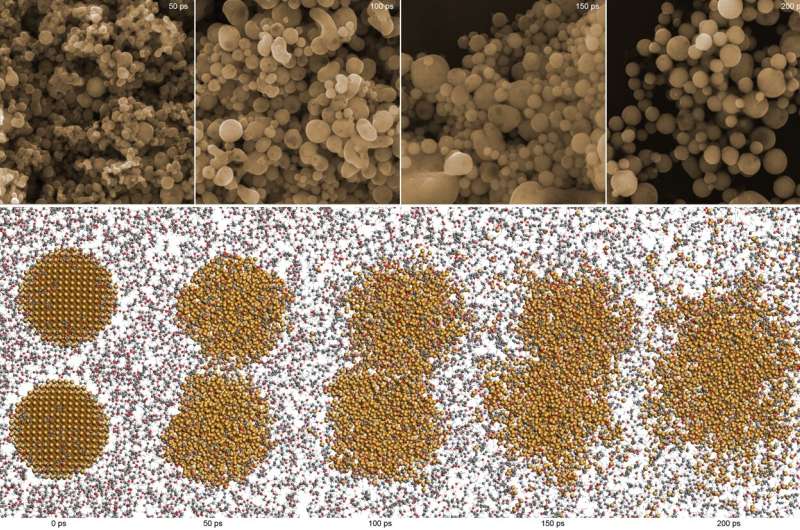
When laser pulses irradiate a suspension of nanoparticles, the particles in the suspension can begin to melt and stick together permanently, while rapidly undergoing chemical reactions that are more or less complex. One of the recent materials obtained in this way, produced at the Institute of Nuclear Physics of the Polish Academy of Sciences (IFJ PAN) in Cracow, turns out to have an unexpectedly high efficiency in catalyzing ethanol, a compound considered to be a promising energy source for fuel cells.
Ethanol is a fuel with many advantages—it can be produced in a renewable manner (for example, from biomass), it can be easily stored as well as having low toxicity. What is of particular importance, however, is the fact that up to several times the amount of electricity can be obtained from a unit mass of ethanol compared to current popular power sources.
Electricity in ethanol-powered fuel cells is generated by processes associated with the oxidation of this alcohol on the catalyst layer of the reaction. Unfortunately, current catalysts do not allow ethanol’s rapid and complete oxidation to water and carbon dioxide. As a result, the cells not only fail to reach maximum efficiency, but also produce undesirable by-products that are deposited on the catalyst and, over time, lead to the disappearance of its properties.
“A considerable obstacle to the commercial success of ethanol cells is also their price. The catalyst we have found can have a significant impact on its reduction and, consequently, on the availability of new cells on the consumer market. This is because its main component is not platinum, but copper, which is almost 250 times cheaper than platinum,” says Dr. Mohammad Shakeri (IFJ PAN), first author of the paper in the journal Advanced Functional Materials.
The achievement of scientists from the IFJ PAN is the result of research conducted on laser control of the size and chemical composition of agglomerates in suspensions. The main idea behind the laser nanosynthesis of composites is the irradiation of a suspension containing agglomerates of nanoparticles of a specific chemical substance with pulses of unfocused laser light with appropriately selected parameters.
The aptly delivered energy causes the temperature of the particles to increase, they melt on the surface and clump together into larger and larger structures, which cool rapidly on contact with the surrounding cool liquid. The temperature reached by the particles is determined by many factors, including the energy of the photons emitted by the laser, the intensity of the beam, the frequency and length of the pulses, and even the size of the agglomerates in suspension.
“Depending on the temperature reached by the agglomerates, various chemical reactions may take place in the material in addition to changes of a purely structural nature. In our research, we focused on the most accurate theoretical and experimental analysis of the physical and chemical phenomena in suspensions in which pulses of laser light were absorbed by nanoparticles of copper and its oxides,” explains Dr. Zaneta Swiatkowska-Warkocka (IFJ PAN).
In the case of real solution particles, the temperature rise occurs in nanoseconds, too rapidly to be measured. In this situation, theoretical molecular dynamics analyses became the first step in understanding the copper systems under study, supported at later stages by simulations performed by the Prometheus computer cluster from Cracow.
Thanks to these, the researchers determined to what temperatures the agglomerates of various sizes would heat up and what compounds might form in these processes. In addition, they checked whether these compounds would be thermodynamically stable or undergo further transformations. The physicists used the knowledge gained to prepare a series of experiments in which nanoparticles of copper and its oxides were laser fused in various proportions.
The composite materials obtained were tested in the laboratories of the IFJ PAN and in the Cracow SOLARIS cyclotron, among others, to determine the degree of oxidation of copper compounds. The information obtained allowed the researchers to identify the optimum catalyst. This turned out to be a three-component system built from appropriate proportions of copper and its oxides of the first and second oxidation state (i.e. Cu2O and CuO).
“From the point of view of efficiency of ethanol catalysis, the crucial discovery was that particles of copper oxide Cu2O3, which is usually thermodynamically very unstable, were present in our material. On one hand, they are characterized by an extremely high degree of oxidation, on the other hand, we found them mainly on the surface of the Cu2O particles, which in practice means that they had very good contact with the solution. It is these Cu2O3 particles that facilitate the adsorption of the alcohol molecules and the breaking of the carbon-hydrogen bonds in them,” states Dr. Shakeri.
Tests on the properties of the catalyst produced by the Cracow physicists ended with optimistic results. The selected composite retained the ability to fully oxidize ethanol even after several hours of use. Moreover, its electrocatalytic efficiency proved comparable to that of contemporary platinum catalysts.
From a scientific perspective, this result is positively astonishing. Catalysis generally proceeds more efficiently the larger the surface area of the agglomerates, which has to do with the fragmentation of their structure. However, the composite studied was not nanometer in size, but several orders of magnitude larger, submicron in size. It seems likely, therefore, that if physicists succeed in reducing the size of the particles in the future, the efficiency of the new catalyst could increase still further.