Researchers from the Department of Chemical Engineering at the Vrije Universiteit Brussel, Riga Technical University and the MESA+ Institute at the University of Twente have succeeded in arranging very small particles (10 µm to 500 nm, 10 to 100 times thinner than a human hair) in a thin layer without using solvents. This is a hugely important first step towards developing a new generation of sensors and electronics for a wide range of applications.
“Common methods based on crystallizing solutions are not as versatile as we would like. What’s more, previous dry methods were mainly effective on sticky surfaces, which limited their applications,” says Ignaas Jimidar of the VUB. To address this issue, the team devised a method of attaching the particles on hard and non-sticky surfaces.
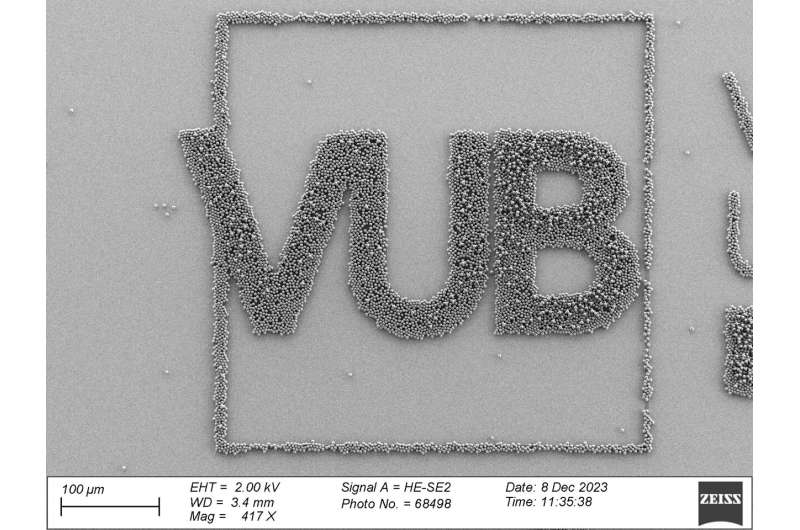
They rubbed the particles across the surface by hand and, within about 20 seconds, achieved a single layer of densely packed particles arranged in a hexagonal pattern.
“The rubbing is done using a stamp made of a silicone-like material called PDMS,” says Kai Sotthewes of the University of Twente. “The static electricity generated by the rubbing process, especially on harder surfaces, and the forces between the particles and the surface are crucial for creating the desired patterns. We encounter this static electricity in everyday life if we rub a balloon against our hair or feel a shock on a dry winter day when we touch a metal object.”
“The pattern-making process worked on both conductive and non-conductive surfaces, and the best results were achieved with certain types of particle powders, such as polystyrene (used as insulation) and polymethyl methacrylate or PMMA, also known as Plexiglas,” says Andris Šutka of Riga Technical University. Silica, a ubiquitous component in contemporary electronics, only worked well on surfaces that were covered with fluorocarbon (a kind of Teflon layer) and when there was no humidity.
“Silica particles are therefore slightly less user-friendly, but they are resistant to all kinds of solvents, which makes them suitable for biological and chemical analysis and detection techniques,” adds Gijs Roozendaal of the University of Twente.
“We eventually succeeded in creating a series of microscopic patterns and logos on ‘wafers’ on a large scale and visualized them all using an atomic force microscope,” says Ignaas Jimidar.
“This represents a promising development for improving electronics, detecting all kinds of chemical and biological substances, and even detecting counterfeit goods. The last is possible because particles in certain patterns refract light differently depending on the angle. So you could detect colors using these microparticles.”
The paper is published in the journal ACS Applied Materials & Interfaces.
Provided by
Vrije Universiteit Brussel


