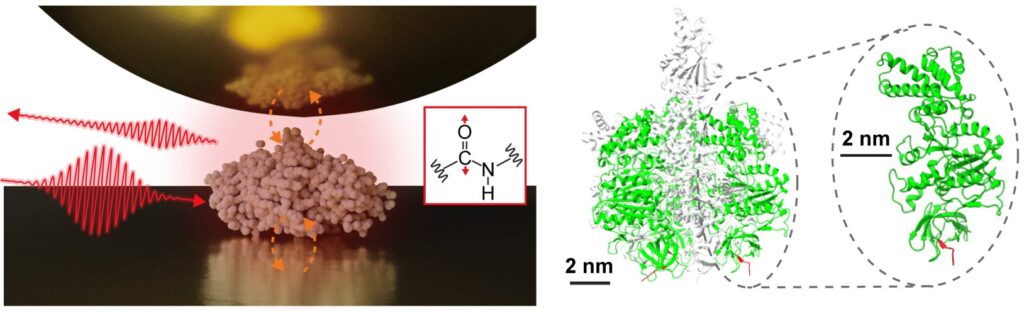
An interdisciplinary research team, led by Assistant Prof. Jun Nishida and Associate Prof. Takashi Kumagai at the Institute for Molecular Science, has successfully observed vibrational spectra of single proteins, consisting of approximately 500 amino acid residues, using advanced measurement techniques based on near-field optical microscopy. This method utilizes light confined at the nanometer scale, allowing for the detailed analysis of extremely small samples, which was previously challenging with conventional infrared spectroscopy.
The study is published in the journal Nano Letters.
Conventional infrared spectroscopy has been widely used for the structural and chemical analysis of various materials as it can measure vibrational spectra, often referred to as the “molecular fingerprints.”
The new achievement represents a major advancement towards technological innovations such as ultra-sensitive and super-resolution infrared imaging, as well as single-molecule vibrational spectroscopy.
The rapid development of nanotechnology in recent years has led to increasing demand for ultra-high sensitivity and super-resolution infrared imaging. However, conventional infrared spectroscopy is limited in measuring extremely small samples or achieving nanometer-scale spatial resolution. For example, even infrared microspectroscopy with good sensitivity requires over a million proteins for obtaining an infrared spectrum, rendering it impossible to measure just a single protein.
In their study, the research team isolated a single protein, a sub-unit comprising a protein complex called F1-ATPase, on a gold substrate and performed near-field infrared spectroscopy measurements in an ambient environment.
They successfully acquired the infrared vibrational spectrum of a single protein, representing a major advance that may lead to characterizing local structural organizations of individual proteins. Such information is particularly important for understanding the sophisticated functions of protein complexes and membrane proteins, offering deeper insights into their mechanisms and interactions.
Furthermore, they have developed a new theoretical framework describing the nanoscale interactions between the infrared near field and protein.
Based on the theory, the team was able to quantitatively reproduce the experimental vibrational spectra that they observed. These results will be invaluable for the chemical analysis of biomolecules as well as various nanomaterials, paving the way for a range of applications of nanoscale infrared spectroscopy.