Hollow-structured supported metal catalysts (i.e. nanoreactor catalysts) with encapsulated active sites and well-defined shells provide an ideal place for multicomponents to react or transform cooperatively in an orderly manner, and efficiently have been recognized as one of the most popular catalyst candidates.
Although reactant enrichment has been proposed by investigating the relationship between the catalytic performance and the structure of nanoreactors at the nano level, the study of the enrichment effect at the mesoscale (500-2000nm) is still not comprehensive enough. Constructing the nanoreactor models with active metals inside and outside the hollow nanostructure via different synthetic methods or sequences will inevitably impact the microenvironment around the active sites, as well as the essential active sites.
Additionally, reactant enrichment at the mesoscale level involves many processes such as adsorption and diffusion, which cannot be elaborated by constructing simple computational models at the nanoscale level. Therefore, investigation of the reactant enrichment at the mesoscale level requires maintaining the intrinsic active sites constant when constructing the research model, either with or without enrichment behavior.
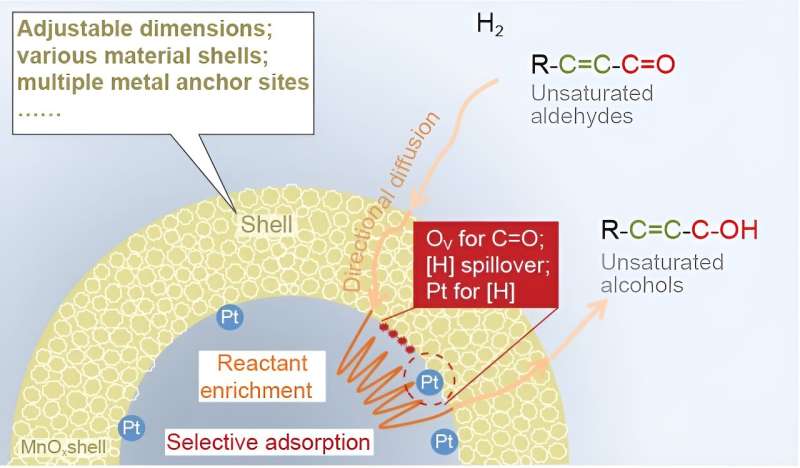
In a new research article published in National Science Review, scientists at Dalian Institute of Chemical Physics (DICP), University of Chinese Academy of Sciences, Taiyuan University of Technology, University of Surrey, and Inner Mongolia University present a new nanoreactor catalyst (Pt NPs@MnOx ) with uniformly dispersed Pt nanoparticles encapsulated in an oxygen vacancy-rich MnOx hollow structure to catalyze the selective hydrogenation of CAL and investigate reactant enrichment at the mesoscale level.
The catalytic performance for CAL-selective hydrogenation on Pt NPs@MnOx is 3.4-fold higher than that of Pt NPs&MnOx, which is physically crushed into an open structure. UV–vis, in situ FTIR and IGA measurements demonstrate that the hollow MnOx shell of Pt NPs@MnOx leads to higher CAL uptake.
The mechanism behind this phenomenon may consist of two steps. As the hollow structure creates a confined space, outer reactants would continuously diffuse into the interior of the hollow structure directionally driven by the concentration gradient and/or capillary-like effect (step 1).
Then, these reactants are fixed on the inner surface by adsorption to keep the local low concentration in the confined space. In contrast, Pt NPs&MnOx could not support this directional diffusion process. Moreover, DFT results reveal that CAL is more strongly adsorbed on the surface of Pt NPs@MnOx than Pt NPs&MnOx under excess reactants (step 2).
H2-TPR–MS and finite-element simulation results also demonstrate that the Pt NPs@MnOx nanoreactor creates a stable space with a high concentration and low flow rate to prevent the escape of the reactants (dissociated hydrogen). It is therefore clear that reactant enrichment is derived from the directional diffusion of reactant driven through a local concentration gradient and an increased amount of reactant adsorbed due to the enhanced adsorption ability in hollow MnOx.
The Pt NPs@MnOx catalyst exhibits extremely high catalytic activities and selectivity in a wide range of reaction pressures. A 95% conversion with 95% COL selectivity is obtained on Pt NPs@MnOx at only 0.5 MPa H2 and 40 min, which is a relatively mild condition compared with most reported catalytic systems.
Combining experimental results with density functional theory calculations, the superior cinnamyl alcohol (COL) selectivity originates from the selective adsorption of CAL and the rapid formation and desorption of COL in the MnOx shell. Moreover, the hollow void induces the reactant-enrichment behavior, enhancing the reaction activity.
These findings offer the possibility of enhancing the catalytic performance at the mesoscale level by designing a rational nanoreactor, rather than reducing the size of the metal particles or modifying them with heteroatoms or ligands at the nanoscale level.