Colloidal gold nanoparticles have been used for a long time by artists because they create vivid colors when they interact with visible light.
These distinct optoelectronic features have recently been explored and used in various high-tech applications, including organic photovoltaics, medical agents, sensory probes, drug delivery in biology and medicine, electronic conductors, and catalysis.
The optical and electronic features of gold nanoparticles can be adjusted by altering their size, surface chemistry, shape, or how they aggregate.
This article from Merck outlines the properties and applications of gold nanoparticles.
Optical and Electronic Properties of Gold Nanoparticles
The optical and electronic characteristics of gold nanoparticles depend significantly on their size, physical dimensions, and surrounding environment. Oscillating electric fields of a light ray interact with colloidal nanoparticles to induce collective oscillation of free electrons that resonate with visible light frequencies.
These resonant vibrations, termed surface plasmons, drive the interaction. In smaller (~30 nm) monodisperse gold nanoparticles, the surface plasmon resonance phenomenon leads to an absorption of light in the blue-green spectrum (~450 nm), while the reflected light, notably red (~700 nm), imparts a vivid red hue.
As the nanoparticle size increases, the absorption linked to surface plasmon resonance shifts toward longer wavelengths, leaning into the red spectrum. Consequently, larger particles absorb red light and reflect blue light, resulting in solutions displaying a faint blue or purple color (Figure 1).
With further size escalation approaching bulk limits, the surface plasmon resonance wavelengths extend into the infrared range of the spectrum, causing the most visible wavelengths to reflect and giving the nanoparticles a transparent or translucent appearance.
Adjusting the size or shape of the nanoparticles allows for fine-tuning the surface plasmon resonance, crafting particles with distinct optical traits suited for various applications.
Figure 1. Colors of various-sized monodispersed gold nanoparticles. Image Credit: Merck
This phenomenon also manifests when surplus salt is introduced into the gold solution: the neutralization of gold nanoparticle surface charge prompts their aggregation, altering the solution’s color from red to blue.
To reduce aggregation, the adaptable surface chemistry of gold nanoparticles permits coating with polymers, biological recognition agents, and small molecules.
Such surface modifications enable gold nanoparticles to be extensively utilized across biological, chemical, engineering, and medical domains. Table 1 outlines the typical properties of these nanoparticles.
Table 1. Product Properties of Gold Nanoparticles. Source: Merck
| Diameter | Nanoparticles /mL |
Peak SPR Wavelength |
Molar Ext (M-1cm-1) |
Product Numbers |
|---|---|---|---|---|
| 5 nm | 5.47 x 1013 | 515-520 nm | 1.10 x 107 | 741949 (Surfactant Stabilized) 752568 (PBS) |
| 10 nm | 5.98 x 1012 | 515-520 nm | 1.01 x 108 | 741957 (Surfactant Stabilized) 752584 (PBS) |
| 15 nm | 1.64×1012 | 520 | 3.67×108 | 777137 (Surfactant Stabilized) 777099 (PBS) |
| 20 nm | 6.54 x 1011 | 524 nm | 9.21 x 108 | 741965 (Surfactant Stabilized) 753610 (PBS) |
| 30 nm | 1.79 x 1011 | 526 nm | 3.36 x 109 | 741973 (Surfactant Stabilized) 753629 (PBS) |
| 40 nm | 7.15 x 1010 | 530 nm | 8.42 x 109 | 741981 (Surfactant Stabilized) 753637 (PBS) |
| 50 nm | 3.51 x 1010 | 535 nm | 1.72 x 1010 | 742007 (Surfactant Stabilized) 753645 (PBS) |
| 60 nm | 1.96 x 1010 | 540 nm | 3.07 x 1010 | 742015 (Surfactant Stabilized) 753653 (PBS) |
| 80 nm | 7.82 x 109 | 553 nm | 7.70 x 1010 | 742023 (Surfactant Stabilized) 753661 (PBS) |
| 100 nm | 3.84 x 109 | 572 nm | 1.57 x 1011 | 742031 (Surfactant Stabilized) 753688 (PBS) |
| 150 nm | 3.60 x 109 | Not Measured | – | 742058 (Surfactant Stabilized) |
| 200 nm | 1.9 x 109 | Not Measured | – | 742066 (Surfactant Stabilized) |
| 250 nm | 7.1 x 108 | Not Measured | – | 742074 (Surfactant Stabilized) |
| 300 nm | 4.5 x 108 | Not Measured | – | 742082 (Surfactant Stabilized) |
| 400 nm | 1.9 x 108 | Not Measured | – | 742090 (Surfactant Stabilized) |
Applications
The applications of gold nanoparticles are expanding swiftly and encompass various fields:
- Electronics – gold nanoparticles are made for use as conductors from electronic chips to printable inks.1 As the size of the electronics world shrinks, these nanoscale gold nanoparticles play a vital role in chip design by linking resistors, conductors, and other chip elements.
- Photodynamic Therapy – Gold nanoparticles that absorb near-infrared radiation light, like gold nanoshells and nanorods, generate heat when stimulated by light within the 700 to 800 nm wavelength range. This allows these nanoparticles to eliminate specific tumors.2 In a method called hyperthermia therapy, gold nanoparticles within a tumor swiftly heat up when light is directed onto them, thus killing tumorous cells.
- Therapeutic Agent Delivery – Therapeutic agents can be coated onto the surface of gold nanoparticles.3 Their extensive surface area-to-volume ratio enables hundreds of molecules—including therapeutics, anti-fouling polymers, and targeting agents—to be coated on their surface.
- Sensors – Gold nanoparticles find use in a diverse range of sensors. For instance, a gold nanoparticle-based colorimetric sensor can determine the suitability of food for consumption.4 Other methods, like surface-enhanced Raman spectroscopy, utilize gold nanoparticles as substrates to measure the vibrational energies of chemical bonds. This technique could also aid in detecting proteins, pollutants, and additional molecules without labels.
- Probes – Gold nanoparticles scatter light and create a range of captivating colors under dark-field microscopy. These colors, created by scattered light from gold nanoparticles, are currently employed in biological imaging.5 Additionally, due to their density, gold nanoparticles serve as valuable probes in transmission electron microscopy.
- Diagnostics – Gold nanoparticles are utilized to identify biomarkers in the diagnosis of cancers, heart diseases, and infectious agents.6 They are also prevalent in lateral flow immunoassays, such as home pregnancy tests.
- Catalysis – Gold nanoparticles serve as catalysts in several chemical reactions.7 The surface of a gold nanoparticle can selectively oxidize or, in some cases, facilitate the reduction of reactions, like nitrogen oxides. Gold nanoparticles are under development for fuel cell applications—potentially beneficial for the automotive and display industries.
Quality Advantage
In partnership with Cytodiagnostics, a comprehensive range of gold nanoparticles is proudly presented, specifically tailored for advanced applications in the life science and materials science fields.
Gold nanoparticles are offered in sizes ranging from 5 nm to 400 nm in diameter, showcasing various surface functionalities across different solvent compositions.
While conventional methods use reducing agents like sodium citrate or sodium borohydride for synthesizing spherical gold nanoparticles, Cytodiagnostics utilizes a proprietary process and formulation, resulting in the creation of highly spherical gold nanoparticles, eliminating the need for harsh reducing agents.
Compared to other gold nanoparticles, these particles offer several benefits including:
- Narrow size distribution – based on Dynamic Light Scattering (DLS) and TEM analysis. Each batch is verified by DLS and UV-Vis spectroscopy (Figure 2).
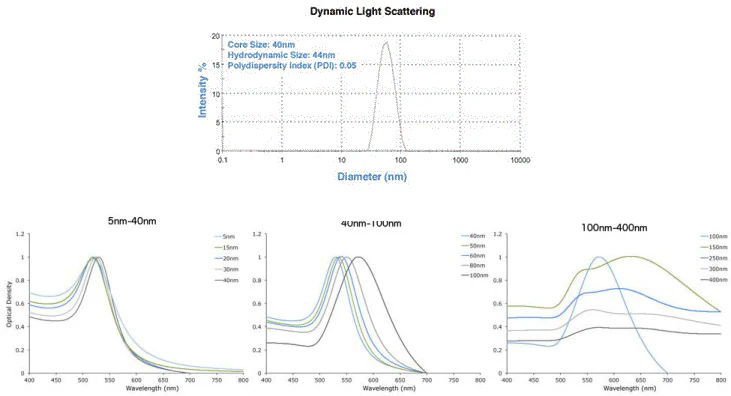
Figure 2. DLS & UV-Vis spectra showing precise gold nanoparticles from Cytodiagnostics. Image Credit: Merck
- Consistent Size and Shape — <10 % CV (coefficient of variance) even above 100 nm. Examples of 5 nm and 400 nm nanoparticles are shown below in Figure 3.
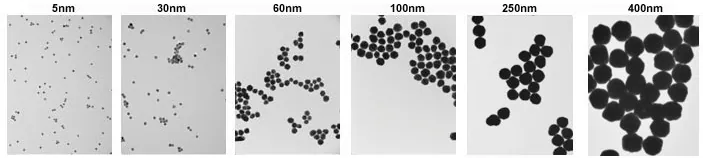
Figure 3. TEM images of 5 nm (left) and 400 nm (right) gold nanoparticles with <8 % CV. Image Credit: Merck
Gold NanoUrchins

Figure 4. TEM of 100 nm Gold NanoUrchins. Image Credit: Merck
Gold NanoUrchins possess distinctive optical properties when compared to spherical gold nanoparticles of the same core diameter. The spiky and irregular surface induces a red shift in the surface plasmon peak and a more substantial enhancement of the electromagnetic field at the tips of the Gold NanoUrchin spikes compared to spherical particles.
For instance, 100 nm spherical gold nanoparticles have a surface plasmon resonance (SPR) peak at 570 nm, whereas 100 nm Gold NanoUrchins exhibit an SPR peak at around 680 nm, as shown in Figure 4.
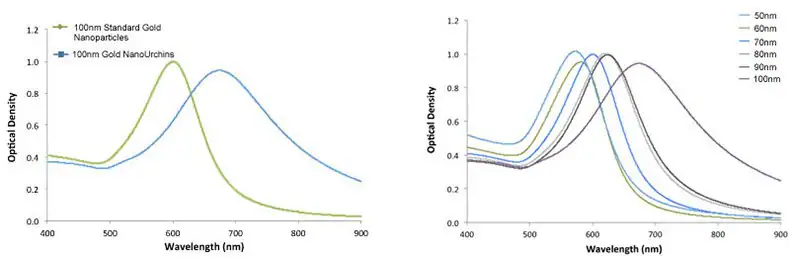
Figure 5. Left – UV-VIS spectra of 100 nm Gold NanoUrchins (blue) and 100 nm standard gold nanoparticles (green). Note the red-shift in the SPR-peak. Right – UV-VIS spectra of Gold NanoUrchins ranging in size from 50 nm to 100 nm in diameter. Image Credit: Merck
Outlook
Gold nanoparticles stand out as versatile materials suitable for a wide array of applications, boasting well-characterized electronic and physical properties due to well-established synthetic procedures. Moreover, their surface chemistry is easily modifiable.
These attributes position gold nanoparticles as one of the most widely utilized nanomaterials in academic research and as an essential component in point-of-care medical devices and industrial products globally.
The extensive offering of gold nanoparticles, accessible to the global research community, aims to foster their adoption in advanced technology applications.
References and Further Reading
- Huang D, Liao F, Molesa S, Redinger D, Subramanian V. 2003. Plastic-Compatible Low Resistance Printable Gold Nanoparticle Conductors for Flexible Electronics. J. Electrochem. Soc. 150(7):G412. https://doi.org/10.1149/1.1582466
- Stuchinskaya T, Moreno M, Cook MJ, Edwards DR, Russell DA. 2011. Targeted photodynamic therapy of breast cancer cells using antibody?phthalocyanine?gold nanoparticle conjugates. Photochem. Photobiol. Sci.. 10(5):822. https://doi.org/10.1039/c1pp05014a
- Brown SD, Nativo P, Smith J, Stirling D, Edwards PR, Venugopal B, Flint DJ, Plumb JA, Graham D, Wheate NJ. 2010. Gold Nanoparticles for the Improved Anticancer Drug Delivery of the Active Component of Oxaliplatin. J. Am. Chem. Soc.. 132(13):4678-4684. https://doi.org/10.1021/ja908117a
- Ali ME, Mustafa S, Hashim U, Che Man YB, Foo KL. 2012. Nanobioprobe for the Determination of Pork Adulteration in Burger Formulations. Journal of Nanomaterials. 20121-7. https://doi.org/10.1155/2012/832387
- Perrault SD, Chan WCW. 2010. In vivo assembly of nanoparticle components to improve targeted cancer imaging. Proceedings of the National Academy of Sciences. 107(25):11194-11199. https://doi.org/10.1073/pnas.1001367107
- Peng G, Tisch U, Adams O, Hakim M, Shehada N, Broza YY, Billan S, Abdah-Bortnyak R, Kuten A, Haick H. 2009. Diagnosing lung cancer in exhaled breath using gold nanoparticles. Nature Nanotech. 4(10):669-673. https://doi.org/10.1038/nnano.2009.235
- Thompson DT. 2007. Using gold nanoparticles for catalysis. Nano Today. 2(4):40-43. https://doi.org/10.1016/s1748-0132(07)70116-0
This information has been sourced, reviewed, and adapted from materials provided by Merck.
For more information on this source, please visit Merck.



