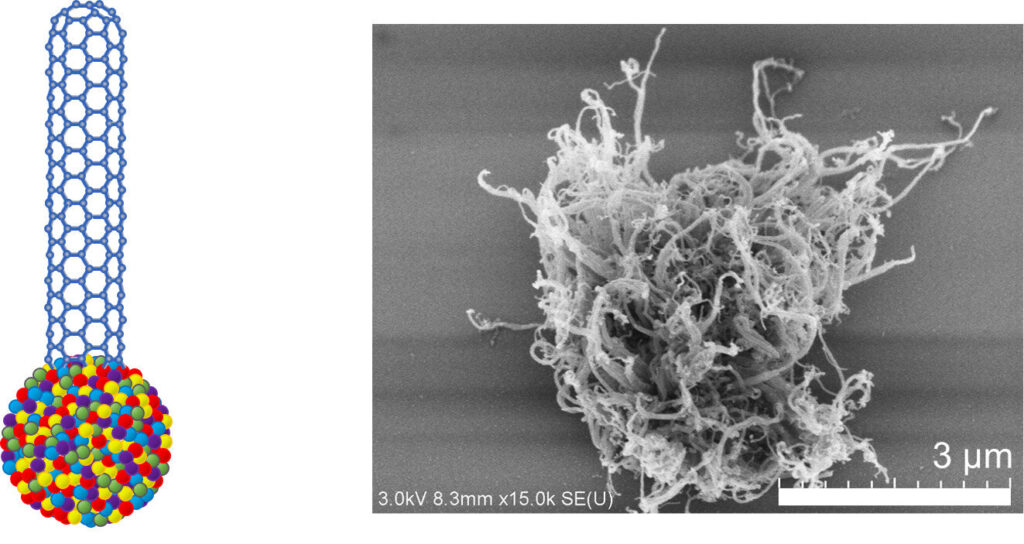
High entropy alloys (HEAs) have attracted significant attention in various fields due to their unique properties such as high strength and hardness, and high thermal and chemical stabilities.
Unlike conventional alloys, which typically incorporate small quantities of one or two additional metals, HEAs constitute a solid solution of five or more metals in equal atomic ratio. This unique composition results in unique and complex surface structures that contain many different active sites suitable for catalytic reactions. As a result, in recent years, HEA nanoparticles (NPs) have been extensively studied for their catalytic potential.
However, despite their potential, HEA NPs have never been used as catalysts for growing single-walled carbon nanotubes (SWCNTs). SWCNTs, nanoscale tubes made up of carbon, exhibit remarkable properties such as exceptional strength and thermal and electrical conductivity, making them valuable in many fields like battery components and biosensors for biomedical and agricultural applications.
Consequently, there is an urgent need for efficient synthesis methods for SWCNTs, necessitating the development of effective catalysts.
In a pioneering study, a team of researchers from Japan, led by Professor Takahiro Maruyama from the Department of Applied Chemistry at Meijo University, achieved, for the first time, growth of SWCNTs using HEA NPs.
“CNTs hold immense potential across numerous domains. If we can reduce their synthesis cost and achieve selective SWCNTs growth through catalyst improvements, it could pave the way for high-speed devices and various optical sensors, making our lives more comfortable,” says Prof. Maruyama.
In previous studies, Prof. Maruyama’s team was successful in growing SWCNTs using single metals such as iridium, platinum, and rhodium as catalysts. Building upon their findings, in this study, they used HEA NPs comprised of five platinum group metals (5 PGM), including rhodium, rubidium, palladium, iridium, and platinum.
Prof. Maruyama explains, “Considering that PGM HEA NPs often have higher activities than individual PGM catalysts, we theorized that HEA NPs composed of PGMs might act as highly active catalysts for growing SWCNTs.”
The team synthesized SWCNTs through the chemical vapor deposition (CVD) process, in which SWCNTs are grown by depositing layers of materials atom by atom on a solid surface in a vacuum. CVD was carried out using acetylene as the feedstock at 750 0C for 10 minutes with the 5 PGM HEA NPs as catalysts. This resulted in the growth of high-density SWNCTs with lengths longer than 1 micrometer. Additionally, Raman analysis showed that the SWNCTs had diameters in the range of 0.83–1.1 nanometers.
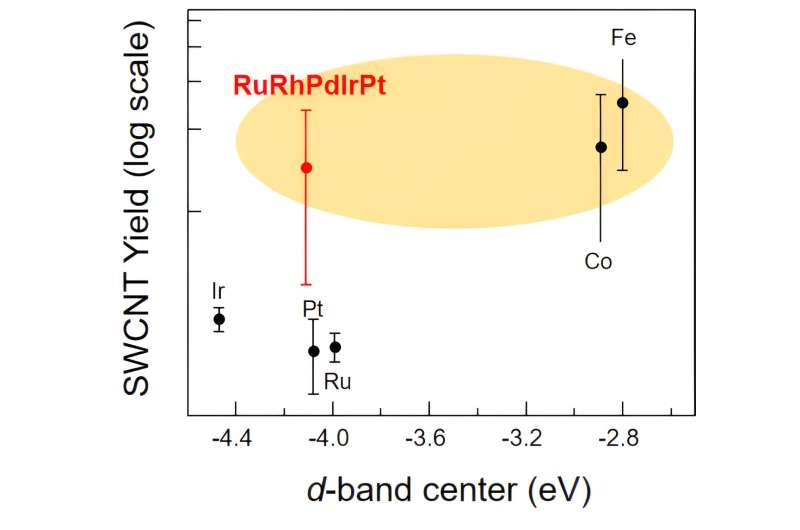
To compare the performance of the HEA NPs, they also synthesized SWNCTs using the individual metals as the catalysts, alongside iron and cobalt, the most commonly used catalysts for obtaining high-yield SWCNTs in the same CVD process. Experiments revealed that the catalytic activity of HEA NPs was considerably higher than that of the individual PGM metals and was comparable to that of iron and cobalt.
The team attributed this high activity to the unique surface structure of HEA NPs that provide various active sites for catalytic reaction owing to the diversity of their atomic structure.
“Our results show that 5 PGM HEA NPs are highly suitable for the growth of small-diameter SWNCTs, representing a completely new matchmaking between materials. Moreover, given the countless combinations possible for HEA composition, our study can pave the way for even superior catalysts,” says Prof. Takamura.
Overall, this study demonstrates the effectiveness of HEA NPs as catalysts for the growth of high-quality SWCNTS, opening new avenues in carbon nanotube research.
The research is published in the journal Applied Physics Express.