Scientists at TU Delft and the Max Planck Institute have made a new class of structurally adaptable ‘mechanical’ pores made from DNA that can transport molecules through cell membranes. These innovative nanopores can open and close on demand and, for the first time, adjust their diameter.
This offers new possibilities for biomedical applications, including controlled and size-selective delivery of macromolecules. The results have been published in Advanced Materials.
Ze Yu, postdoc in Sabina Caneva’s group and co-first author of the publication, explains that DNA origami nanopores are widely used in biophysics and biotechnology to analyze protein shapes and compositions. However, traditional pores are too narrow for macromolecules such as therapeutics to pass through, and the pores are constantly open, which is not ideal for targeted drug delivery.
Structurally adaptable pores
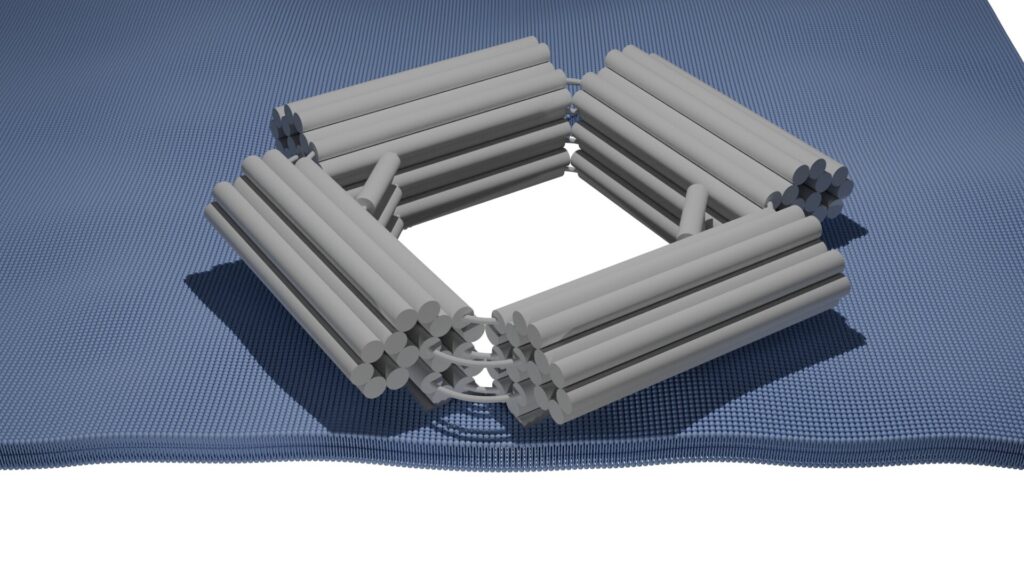
Caneva and her team, in collaboration with the Heuer-Jungmann lab at Max Plack Institute of Biochemistry, have designed and developed nanopores with a wider opening of 30 nanometers (MechanoPores), instead of the usual 4–5 nanometers.
“DNA is an ideal material for building on a small scale,” says Yu. “We use the hydrogen bonds between complementary base pairs to create the desired structure with DNA strands.” With this approach, DNA origami nanotechnology can be used to build precise, pre-programmed 2D and 3D shapes.
The biggest challenge was making the pores open and close on-demand. Caneva’s team used the flexible properties of single-stranded DNA to achieve this, essentially resembling a compliant mechanism. Inside the pore, there is a flexible single-stranded DNA molecule on two sides.
When a complementary DNA strand is added, a stiffer double-stranded DNA molecule forms, pushing the pore open and allowing larger biomolecules to pass through. To close the pore, single-stranded DNA molecules which are complementary to the DNA molecules on the outside of the pore are added, forcing the pore to close.
Size tunability
According to Yu, this is the first nanopore that can reversibly adopt three different diameters and can therefore select molecules based on size.
The research shows that the pore can also be efficiently actuated in a membrane, something scientists have not achieved before. This requires a biochemical trick to coax the MechanoPore to sit across the biomembrane, and was confirmed via fluorescence imaging of molecular flow through the pore.
Sabina Caneva, assistant professor at the Faculty of Mechanical Engineering, said, “Our work is an important step towards more advanced dynamic nanodevices with potential uses in the field of controlled drug delivery and molecular diagnostics, where controlled transport of biological macromolecules through large, stable channels is crucial.”
The next step is to select molecules not only by size but also by molecular composition. “There are different proteins of roughly the same size. Our aim is to differentiate between these proteins based on molecular composition for even more selective transport through the nanopore,” says Yu.