Researchers in Purdue University’s College of Engineering have invented and are developing noninvasive medical devices to make the monitoring and treatment of certain physiological and psychological conditions timelier and more precise.
Wenzhuo Wu, the Ravi and Eleanor Talwar Rising Star Associate Professor of Industrial Engineering, said noninvasive, repeated monitoring of uric acid (UA) levels in human sweat over long periods of time could enable the unprecedented diagnosis, therapy, and prognosis of several conditions, including anxiety and hypertension.
“My team and I have created new noninvasive, wearable sensors that monitor levels of uric acid in human sweat,” Wu said. “These patent-pending sensors, called EPICS, have higher sensitivity and better wearability and can be made from less expensive materials than traditional sensors that measure uric acid levels.”
A paper about the research has been published in Nano Energy.
The impact of uric acid
Wu said UA is made in the human body as an end product of purine metabolism. It also acts as an alarm that triggers inflammation as an immune response.
“Variation in UA concentration could indicate physiological diseases such as gout, hyperuricemia, and hypertension, as well as psychological conditions such as anxiety and depression,” Wu said.
“Recent studies report the physiological diseases associated with abnormal UA levels affect approximately 1%-4% of the world’s population and cost more than $20 billion in annual medical expenditures. The psychological conditions associated with abnormal UA levels impact 8.74% of the U.S. population and cost $33.7 billion in related medical expenses annually.”
Drawbacks of traditional uric acid monitoring
Wu explained there are well-established clinical measures of UA levels in blood used for metabolism and nutrition control. He also said they have drawbacks.
“The intrusive nature of collecting blood and the delay between sample collection and analysis are major hindrances, especially to personalized remote treatments like flare-up prevention and just-in-time nutrition control,” Wu said. “Monitoring UA levels in sweat samples has the advantages of being noninvasive and offering real-time results.”
Wu said current wearable sensors to measure UA levels in sweat have several limitations, including complicated fabrication processes, sophisticated instruments, expensive raw materials, and unsatisfactory performance.
“The UA levels in the sweat of a healthy human are significantly lower than the UA levels in blood. This means sensors must have superior limits of detection,” Wu said. “Additionally, continuous monitoring requires intimate contact between the UA sensor and human skin, which imposes further requirements for the wearability of the sensors.”
EPICS sensors
Wu and his team have developed EPICS, which are flexible and noninvasive sensors that monitor uric acid in human sweat. They created the sensors from zinc oxide, a nontoxic, biocompatible, and electrochemically active material.
“Our design allows the possibility of noninvasive monitoring of UA with a boosted performance by otherwise wasted mechanical energy, such as that from the human body,” Wu said. “The fundamental piezo-electrocatalytic principles can also be extended to other piezoelectric materials with catalytic properties for high-performance sensing in the biomedical, pharmaceutical, and agricultural areas.”
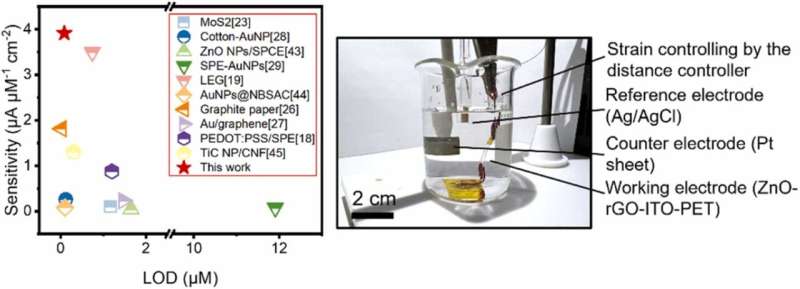
Wu and his team have tested EPICS at Purdue University’s Flex Lab since the summer of 2021. He said the results show EPICS outperformed traditional UA sensors in the tests.
“We demonstrated that the EPICS devices achieve a fourfold enhancement in the UA sensing performance with a small compressive strain boosted by piezo-electrocatalysis during the electrochemical oxidation of UA on the surfaces of mechanically deformed zinc oxide nanorods,” Wu said. “The EPICS devices exhibited a superior sensitivity and limit of detection outperforming all reported flexible electrochemical UA sensors.”
Wu and the research team will conduct additional testing to validate the on-body sensing of EPICS and to evaluate the sensor’s performance over time.