Griffith University researchers have developed innovative, eco-friendly quantum materials that can drive the transformation of methanol into ethylene glycol.
Ethylene glycol is an important chemical used to make polyester (including PET) and antifreeze agents, with a global production of over 35 million tons annually with strong growth.
Currently, it’s mainly produced from petrochemicals through energy-intensive processes.
Methanol (CH3OH) can be produced sustainably from CO2, agricultural biomass waste, and plastic waste through various methods such as hydrogenation, catalytic partial oxidation, and fermentation. As a fuel, methanol also serves as a circular hydrogen carrier and a precursor for numerous chemicals.
Led by Professor Qin Li, from Griffith’s Queensland Micro- and Nanotechnology Center, the team’s method uses solar-driven photocatalysis to convert methanol into ethylene glycol under mild conditions. This process uses sunlight to drive chemical reactions, which minimizes waste and maximizes the use of renewable energy.
The study, “Colloidal Synthesis of Carbon Dot-ZnSe Nanoplatelet van der Waals Heterostructures for Boosting Photocatalytic Generation of Methanol-Storable Hydrogen,” has been published in the journal Small.

While previous attempts at this conversion have faced challenges—such as the need for toxic or precious materials—Professor Li and the research team have identified a greener solution.
“Climate change is a major challenge facing humanity today,” Professor Li said.
“To tackle this, we need to focus on zero-emission power generation, low-emission manufacturing, and a circular economy. Methanol stands out as a crucial chemical that links these three strategies.
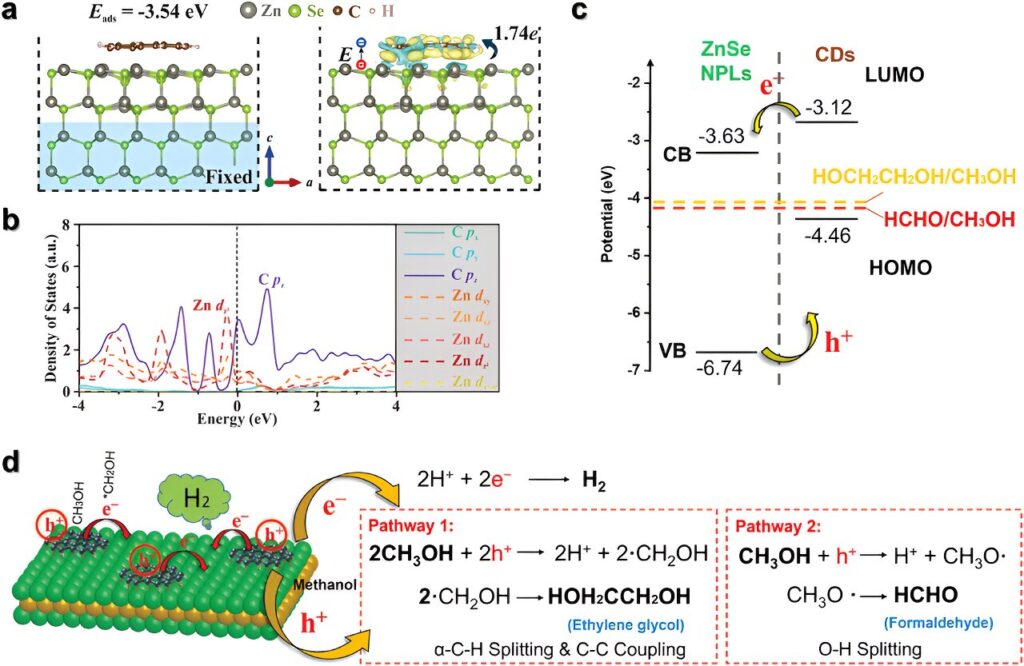
“What we have created is a novel material that combines carbon quantum dots with zinc selenide quantum wells.”
“This combination significantly enhances the photocatalytic activity more than four times higher than using carbon quantum dots alone, demonstrating the effectiveness of the new material,” Lead author Dr. Dechao Chen said.
The approach has also shown high photocurrent, indicating efficient charge transfer within the material, crucial for driving the desired chemical reactions.
Analyses confirmed the formation of ethylene glycol, and the byproduct of this reaction is green hydrogen. This discovery opens up new possibilities for using eco-friendly materials in photocatalysis, paving the way for sustainable chemical production.
As a new quantum material, it also has the potential to lead to further advancements in photocatalysis, sensing, and optoelectronics.
“Our research demonstrates a significant step towards green chemistry, showing how sustainable materials can be used to achieve important chemical transformations,” says Professor Qin Li. “This could transform methanol conversion and contribute significantly to emissions reduction.”