As a combustible fuel, the burning of hydrogen gas does not contribute to global warming. Today, the majority of hydrogen gas is generated from fossil fuels, however, and this process releases greenhouse gases into the atmosphere. Generating hydrogen gas from clean sources, such as the splitting of water molecules with electricity through electrolysis, is important to achieving future carbon neutrality, but current methods are inefficient and limit the commercial practicality of hydrogen-based technologies.
A new electrocatalyst leverages enhanced electrochemical activity, reaction surface area and durability to improve the efficiency of hydrogen gas production via electrolysis.
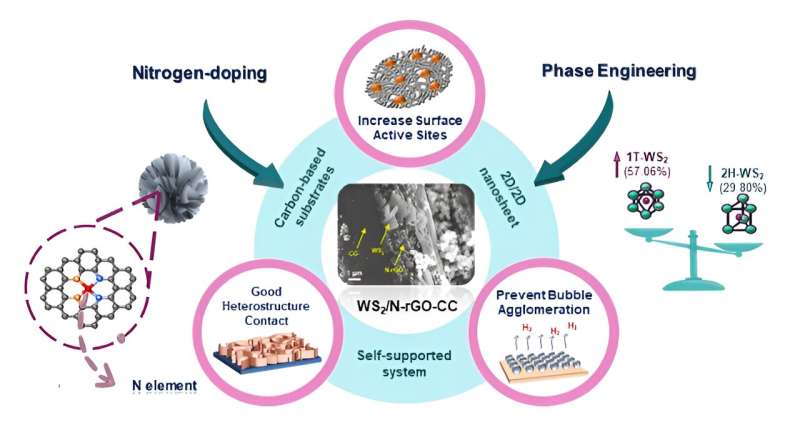
Researchers from Center of Excellence for NaNo Energy & Catalysis Technology (CONNECT), Xiamen University in Malaysia synthesized and characterized an efficient and durable water electrocatalyst composed of the transition metal dichalcogenide tungsten disulfide (WS2), a two-dimensional material with semiconducting properties, that functions as an electron acceptor or donor in the electrolysis reaction.
The electrocatalyst, WS2/N-rGO/CC, is created on a carbon cloth (CC) that is bound to reduced graphene oxide (rGO), a two-dimensional lattice semiconductor, combined with a very small amount of nitrogen (N) to alter the properties of the reduced graphene oxide semiconductor. A hydrothermal reaction converts two-dimensional WS2 into microscopic, three-dimensional flower-like structures called nanoflowers that increase the surface area of the electrocatalyst to improve reaction efficiency.
The team published their results in the journal Nano Research.
“Synthesizing a self-supported electrode for the hydrogen evolution reaction in water hydrolysis is crucial because it addresses a fundamental challenge in clean energy production. Traditional methods often rely on expensive catalysts and supports, which can limit the efficiency and scalability of hydrogen production. Our work represents a significant advancement by creating a self-supported electrode that not only enhances the electrocatalytic activity, but also offers a cost-effective and sustainable solution for hydrogen generation,” said Feng Ming Yap, lead author of the paper and graduate student in the School of Energy and Chemical Engineering at Xiamen University Malaysia in Selangor Darul Ehsan, Malaysia.
Because the active species of the electrocatalyst, tungsten disulfide, is directly incorporated into the conductive materials of the electrode, WS2/N-rGO/CC is considered a self-supported electrode. No polymer binders or additives are present in the synthesized electrocatalyst to mask catalyst active sites or decrease electron conductance, maximizing reaction efficiency.
The research team experimented with incorporating various amounts of dimethylformamide (DMF) in the final hydrothermal synthesis reaction to determine the best concentration for the preferred metallic 1T phase transition of WS2 for the electrode. The electrode developed using a 50% concentration of DMF in water (50% WGC) during the last hydrothermal reaction demonstrated superior characteristics to electrodes synthesized using 0, 25, 75 and 100 percent DMF solutions.
“Our electrode can efficiently produce hydrogen under a wide range of pH conditions, making it versatile and adaptable for various practical applications. It is a step towards sustainable and efficient hydrogen production, which is essential for a cleaner energy future,” said Wee-Jun Ong, supervisor of the project and associate professor in the School of Energy and Chemical Engineering at Xiamen University Malaysia.
Importantly, the 50% WGC electrocatalyst outperformed the platinum benchmark electrocatalyst, 20% Pt-C/CC, for the HER in both acidic and basic conditions. Specifically, 50% WGC demonstrated a lower overpotential, or energy required to split water, than 20% Pt-C/CC. The overpotential for 50% WGC was 21.13 mV compared to 46.03 mV for 20% Pt-C/CC.
The research team believes that more cost- and energy-efficient electrocatalysts, like 50% WGS, are paramount to achieving the world’s clean energy goals. “We aim to explore the scalability and practical implementation of our self-supported electrode technology. Our ultimate goal is to contribute to the transition to a sustainable energy landscape, where hydrogen can play a crucial role as a clean and renewable energy source,” said Ong.
Jian Yiing Loh from the School of Energy and Chemical Engineering and the Center of Excellence for NaNo Energy & Catalysis Technology (CONNECT) at Xiamen University Malaysia in Selangor Darul Ehsan, Malaysia also contributed to the study. This research is part of the initiatives of the national policies in Malaysia, namely National Energy Transition Roadmap (NETR), and Hydrogen Economy and Technology Roadmap (HETR), to facilitate Malaysia’s sustainable energy in the next five years.
Provided by
Tsinghua University Press