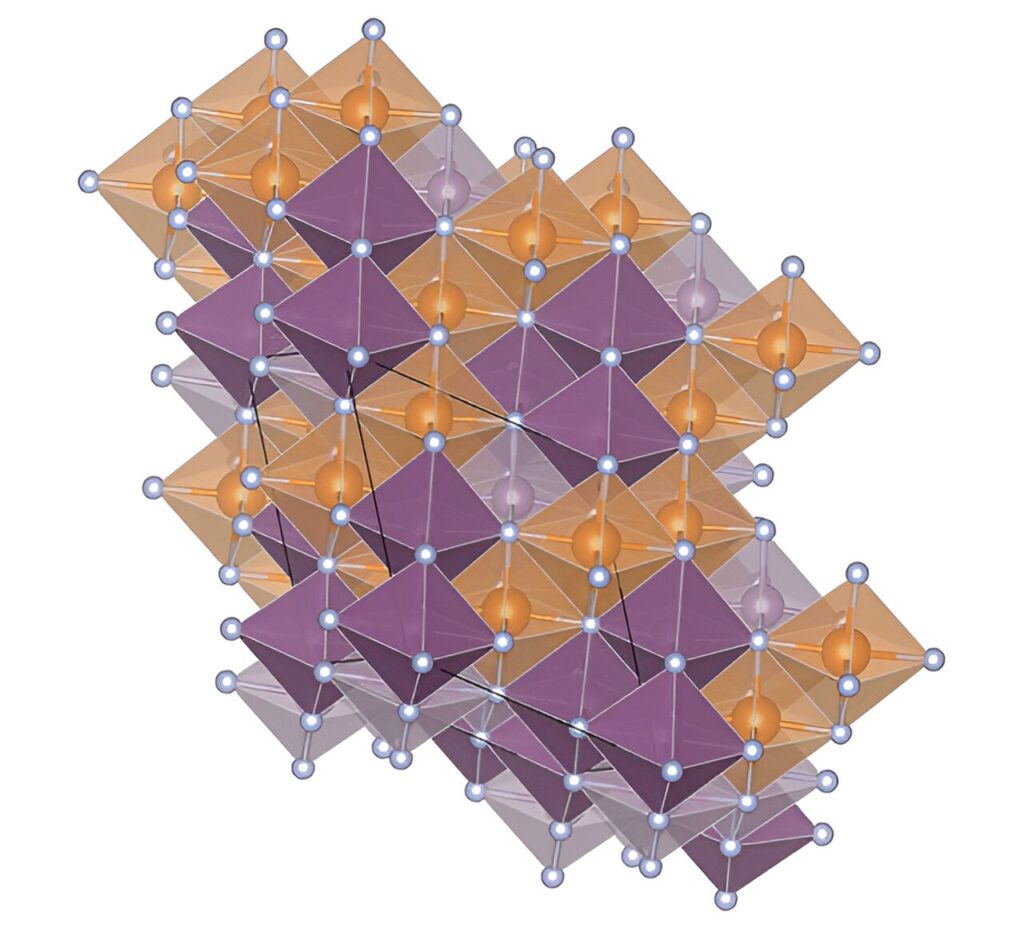
A research team discovered a method to transform materials with three-dimensional atomic structures into nearly two-dimensional structures—a promising advancement in controlling their properties for chemical, quantum, and semiconducting applications.
The field of materials chemistry seeks to understand, at an atomic level, not only the substances that comprise the world but also how to intentionally design and manufacture them.
A pervasive challenge in this field is the ability to precisely control chemical reaction conditions to alter the crystal structure of materials—how their atoms are arranged in space with respect to each other. Controlling this structure is critical to attaining specific atomic arrangements that yield unique behaviors. This process results in novel materials with desirable characteristics for practical applications.
A team of researchers led by the National Renewable Energy Laboratory (NREL), with contributions from the Colorado School of Mines (Mines), National Institute of Standards and Technology, and Argonne National Laboratory, discovered a method to convert materials from their higher-energy (or metastable) state to their lower-energy, stable state while instilling an ordered and nearly two-dimensional arrangement of atoms—a feat that has the potential to unleash promising material properties.
The researchers published their findings in a paper titled “Synthesis Pathways to Thin Films of Stable Layered Nitrides,” in Nature Synthesis.
“A compelling reason to find ways to produce stable thin films with layered, nearly two-dimensional structures is that many of them have unusual chemical, semiconducting, or quantum properties. This is because electrons in such two-dimensional materials interact only with other electrons sideways—not above or below,” said NREL’s Andriy Zakutayev, senior physics researcher who synthesized the materials and led this study.
“These two-dimensional properties could be promising for practical applications, such as electrocatalysts for hydrogen production, energy-efficient electronic devices, or superconducting qubits for quantum computing.”

Understanding the formation of disordered metastable phases
Nitrides are nitrogen-containing chemical compounds that can form robust materials. They are known for their chemical resistance and thermal stability, and these properties make them indispensable in high-performance industrial applications, especially in thin films that are often only a few atoms thick. Common applications for these films include use as semiconductor insulation layers and as protective coatings for optical lenses and machining tools.
However, the process of creating a thin nitride film tends to produce molecular structures that are three-dimensional and not fully stable. To achieve nitrides with the stable two-dimensional layered structures that are useful for chemical or quantum applications, NREL researchers examined why these intermediate phases form at all.
When a compound’s constituent atoms reach low-energy areas—called local minima—the compound tends to settle into that structure. The regions from which an atom will move toward these local minima are called basins of attraction. Compounds with stable structures that have smaller basins of attraction are more likely to be stuck in a metastable state—between stability and instability.
“From a theoretical perspective, the larger the basin of attraction, the more likely it is that a compound will settle into that arrangement, which is why three-dimensional metastable nitrides form—like rainwater flowing into a large puddle formed in a big pothole on the road,” said Mines’ Vladan Stevanovic, associate metallurgical and materials engineering professor who performed the study’s theoretical calculations with his team of students.
“Here, we discovered how certain metastable three-dimensional structures might change into stable, nearly two-dimensional layered structures. This is exciting—it’s like finding a space wormhole in science fiction.”
Discovering a pathway to achieve thin films of stable layered nitrides
The team synthesized thin nitride films with magnesium and molybdenum by radio frequency sputtering—a procedure in which the precursor metals are blasted with energetic ions, removing atoms that will form thin films—in an atmosphere of argon and nitrogen. The new compounds were then subjected to a rapid heat treatment process under an atmospheric nitrogen environment.

“The experimental observations indicate that the compounds, as deposited, crystallize into a three-dimensional, metastable cubic structure with elemental disorder,” Zakutayev said.
“But when we applied heat above 700°C (1,292°F), the compounds transformed into nearly two-dimensional thin films with hexagonal structure with elemental order. We were quite surprised by the emergence of the order from disorder—it was like throwing together mixed pasta, cheese, and veggies all together into a pan and then taking it out of an oven and finding a delicious, layered lasagna there.”
The key to solving this mystery was an elemental order hidden on the very short atomic length scale in the otherwise disordered metastable materials. The team validated this discovery with three other nitride materials and two independent experimental measurements in addition to theoretical calculations.
Implications of a thin-film transformation pathway
Beyond the specific compounds in the team’s experiments, the team’s discovery is also applicable to other nitride thin films that are only known to form three-dimensional cubic structures. Control over a material’s final atomic structure is essential to changing that material’s properties.
This is especially true for materials with quantum properties that respond rapidly to slight changes in atomic structure and for materials with semiconductor properties that are adjustable with atom rearrangement.
“Our team was able to synthesize three other nitride compounds in a layered, nearly two-dimensional structure using this same method, demonstrating the universality of our approach,” said NREL’s Rebecca Smaha, materials science researcher who performed synchrotron measurements.
“We also developed a theoretical explanation for how these materials can be synthesized, making this synthesis method suitable for other chemistries beyond nitrides. I’m excited to see how this synthesis pathway can be leveraged to discover completely new materials in inorganic solid-state materials chemistry.”