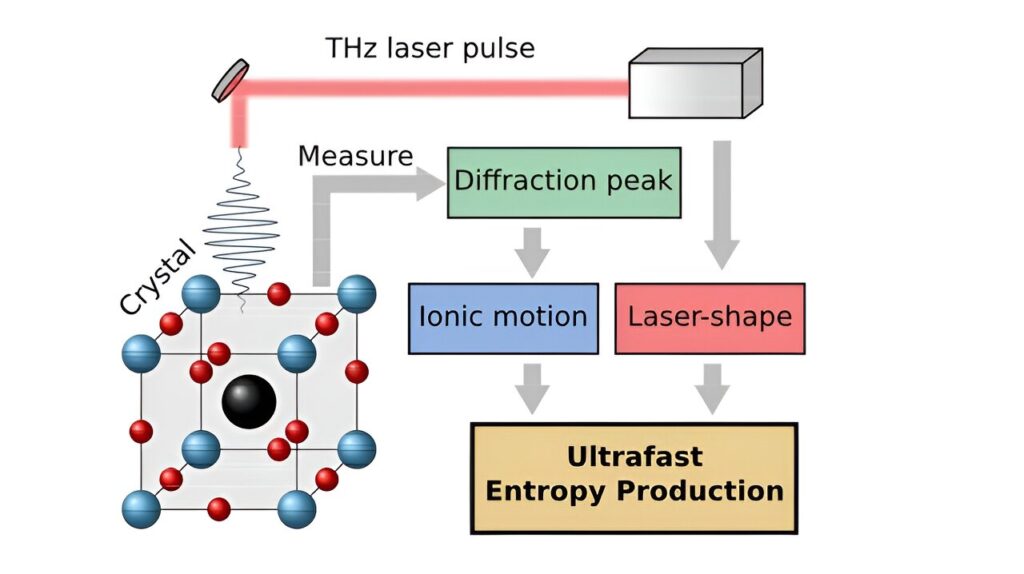
Entropy, the amount of molecular disorder, is produced in several systems but cannot be measured directly. An equation developed by researchers at Chalmers University of Technology in Sweden, and Heinrich Heine University Düsseldorf, now sheds new light on how entropy is produced on a very short time scale in laser excited materials.
“New computational models give us new research opportunities. Extending thermodynamics for ultrashort excitations will provide novel insights into how materials function on the nanoscale,” says Matthias Geilhufe, Assistant Professor at the Department of Physics at Chalmers University of Technology.
Entropy is a measure of irreversibility and disorder and is central in thermodynamics. Two centuries ago, it was part of a conceptual breakthrough, building the theoretical framework for machines, fundamental for the industrial revolution. Today, we are seeing advances in new areas of nano and quantum devices, but still, entropy is a pivotal concept.
“A system usually wants to evolve to a state with large disorder, i.e. maximum entropy. It can be compared to a sugar cube dissolving in a glass. While the sugar dissolves, the system composed of water and sugar slowly increases its entropy. The reverse process—a spontaneous formation of a sugar cube—is never observed,” says Matthias Geilhufe.
A computational model for entropy
“If we turn to how entropy is formed in devices, they all need to be turned on and off, or need to move something from A to B. As a consequence, entropy is produced. In some cases, we would like to minimize the entropy production, for example to avoid information loss,” says Matthias Geilhufe.
While entropy has become a well-established concept, it cannot be measured directly. However, Matthias Geilhufe together with researchers Lorenzo Caprini and Hartmut Löwen at Heinrich Heine University Düsseldorf, have developed a computational model to measure entropy production on a very short time scale in laser excited crystalline materials. Their paper, “Ultrafast entropy production in pump-probe experiments,” was published in Nature Communications.
Phonons in crystalline materials can produce entropy
Crystalline materials are essential for various technologies that transfer and store information over short periods, such as semiconductors in computers or magnetic storage spaces. These materials are made up of a regular crystalline lattice, whereby atoms arrange in repeating patterns.
Laser light can shake the atoms into a collective motion which physicists call phonons. Astonishingly, phonons often behave as if they were a particle. They are called quasiparticles, to distinguish them from actual particles like electrons or ions.
What the researchers have now discovered, is that the phonons—the lattice vibrations in the crystalline materials—can produce entropy in the same way as bacteria in water as shown by previous research in biological physics by Caprini and Löwen.
By the very nature of the phonon being a quasiparticle in a crystal it can be shown that the same mathematical pattern holds as for their biological counterparts in water. This insight precisely determines the entropy and heat production in laser excited materials and allows us to understand or even change their properties on demand.
The researchers’ computational model can also be applied to other types of material excitations and thus opens a new perspective in the field of research on ultrafast materials.
“In the long run, this knowledge can be useful for tailoring future technologies, or lead to new scientific findings,” says Matthias Geilhufe.