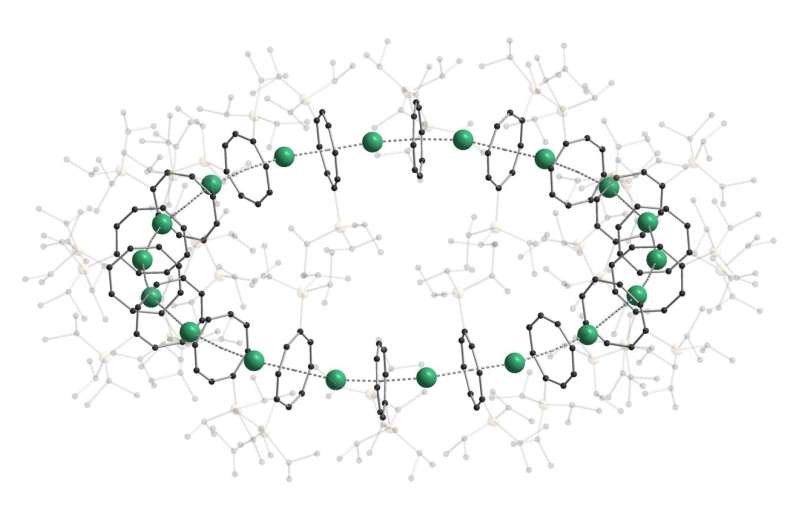
Sandwich compounds are special chemical compounds used as basic building blocks in organometallic chemistry. So far, their structure has always been linear.
Recently, researchers of Karlsruhe Institute of Technology (KIT) and the University of Marburg were the first to make stacked sandwich complexes form a nano-sized ring. Physical and other properties of these cyclocene structures will now be further investigated. The researchers report their findings in Nature.
Sandwich complexes were developed about 70 years ago and have a sandwich-like structure. Two flat aromatic organic rings (the “slices of bread”) are filled with a single, central metal atom in between. Like the slices of bread, both rings are arranged in parallel. Adding further layers of “bread” and “filling” produces triple or multiple sandwiches.
“These compounds are among the most important complexes used in modern organometallic chemistry,” says Professor Peter Roesky from KIT’s Institute for Inorganic Chemistry. One of them is the highly stable ferrocene, for which its “fathers” Ernst Otto Fischer and Geoffrey Wilkinson were awarded the Nobel Prize in Chemistry in 1973. Ferrocene consists of an iron ion and two five-membered aromatic organic rings. It is used in synthesis, catalysis, electrochemistry, and polymer chemistry.
First nano-sized rings
For some time now, researchers of KIT and the University of Marburg have tried to arrange sandwich complexes in a ring. “We succeeded in producing chains, but no rings,” Roesky says, who coordinated the work of three teams at the two universities. “Thanks to the choice of the right ‘slice of bread’ or organic intermediate deck, we have now succeeded in forming nano-sized rings for the first time,” say Professor Manfred Kappes, who heads the Division of Physical Chemistry of Microscopic Systems at KIT, and Professor Florian Weigend, Head of the Applied Quantum Chemistry Unit of the University of Marburg.
The new nanoring consists of 18 building blocks and has an outer diameter of 3.8 nanometers. Depending on the metal used as the “filling” of the sandwich, an orange-colored photoluminescence results. The new chemical compound was called “cyclocene” by the researchers.
The nanoring is held together by itself
The three working groups carried out elaborate quantum chemical calculations to find out why the molecules could be arranged in a ring and no longer formed a chain of sandwich complexes. These calculations revealed that the driving force for the ring formation is the energy gained by the ring closure.
“Our challenge initially was to form a ring. Can other ring sizes be produced? Does this nanostructure possess unusual physical properties? This will be subject of further research. But it is clear now that we have added a new building block to our toolbox of organometallic chemistry. And this is great,” Roesky says.