Researchers from Tokyo Metropolitan University have created a new drug-delivery molecule, a zwitterionic polymer complex that can help get plasmid DNA inside cells when injected into skeletal muscle, a crucial step in the expression of therapeutic RNA and proteins. The study is published in the journal Biomaterials Science.
The new compound is effectively bound to plasmid DNA without affecting its structure. Injected into mouse muscles, the team observed widespread gene expression.
Drug-delivery systems underpin many of the clinical breakthroughs of our age. For example, the COVID-19 vaccine uses lipid nanoparticles to encase messenger RNA (mRNA) and carry them into cells by a process called endocytosis; once inside, mRNA is released via “endosomal escape” before it is “translated” by cellular machinery into antigens which provoke an immune response.
But while such methods have been successfully used, there are still challenges to be overcome, like unwanted aggregation of the carrier. As treatments diversify, researchers are on the lookout for new delivery methods for a wider range of applications.
A team from Tokyo Metropolitan University led by Professor Shoichiro Asayama have been studying the use of polyions, polymers with an electric charge, to carry plasmid DNA (pDNA) into cells.
Plasmid DNA can be transcribed to messenger RNA or translated into proteins, making them a versatile vehicle for therapies. They also happen to be negatively-charged polymers which can bind to positively charged polyions.
However, simply making a large, positively-charged polymer is far from ideal, since their charge might make them toxic to cells. Recent efforts have turned to zwitterions, that is, compounds with a positive charge on one part and a negative charge on another.
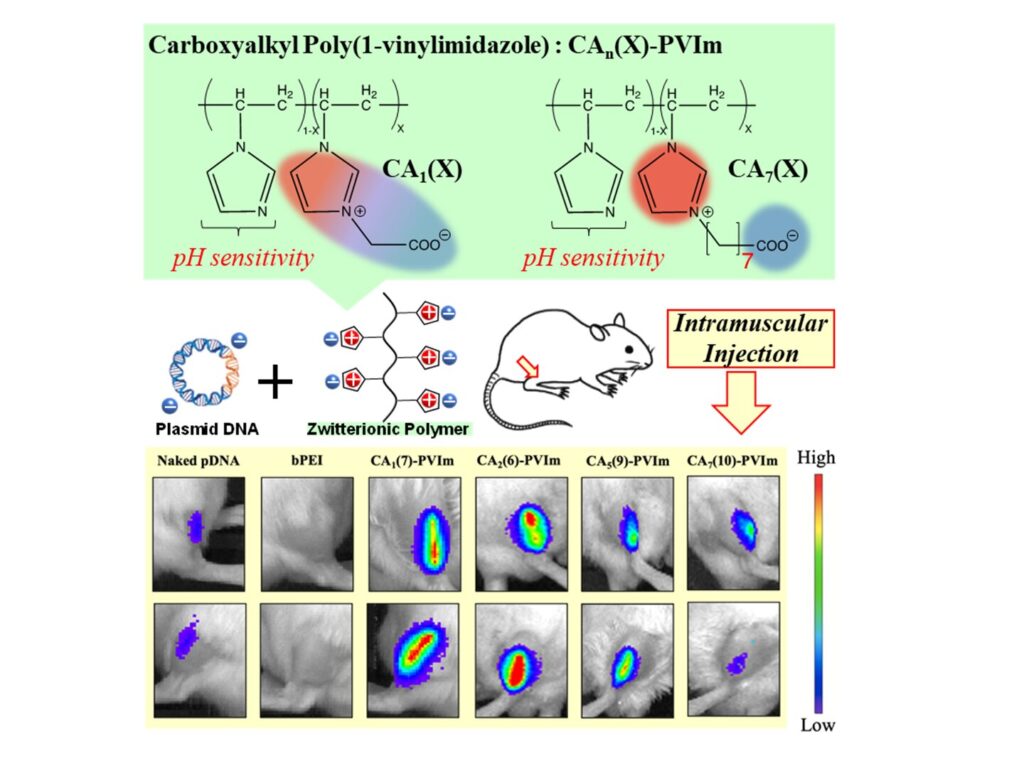
Now, the team have engineered the first zwitterionic polymer compound (CA-PVIm) with an imidazolium cation (positive charge) which can complex with pDNA.
Imidazolium groups have the advantage of having positive charge smeared out over a ring of atoms, giving them a good chance of binding strongly to pDNA. Negatively-charged portions were composed of carboxyl groups spaced out by a short hydrocarbon chain; these were added into the polymer chain in different proportions.
In preliminary experiments, they found that their new compound had a layer of bound water molecules in solution which might render them bioinert. Mixed with pDNA, a method used to separate DNA compounds by length was used to show that pDNA can successfully complex with CA-PVIm. Other measurements also demonstrated that the complex hierarchical structure of the pDNA was preserved.
The team put their compound to the test by injecting it into the muscle tissue of mice. Compared to bare pDNA, they found gene expression due to the pDNA over a drastically wider area.
This clearly showed that their polyion was being taken up into cells and undergoing endosomal escape. They also identified an optimal compound, with 7% of available sites given negative charges (CA(7)-PVIm), that gave the greatest effect.
Since it can deliver its cargo over large masses of muscle, the team’s findings promise new therapies for serious muscular diseases.