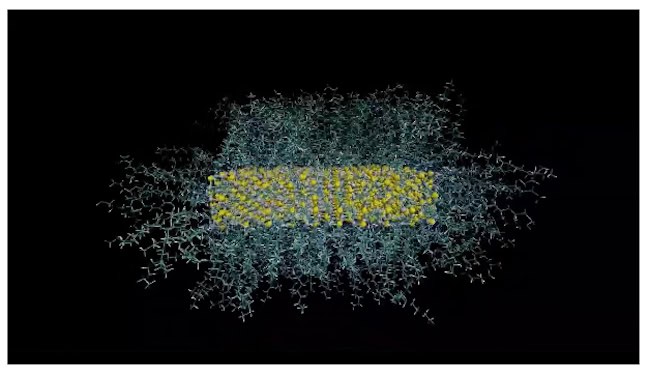
Molecules known as ligands attach more densely to flatter, platelet-shaped semiconductor nanocrystals than they do to spherical ones – a counterintuitive result that could lead to improvements in LEDs and solar cells as well as applications in biomedicine. While spherical nanoparticles are more curved than platelets, and were therefore expected to have the highest density of ligands on their surfaces, Guohua Jia and colleagues at Australia’s Curtin University say they observed the exact opposite.
“We found that the density of a commonly employed ligand, oleylamine (OLA), on the surface of zinc sulphide (ZnS) nanoparticles is highest for nanoplatelets, followed by nanorods and finally nanospheres,” Jia says.
Colloidal semiconductor nanocrystals show promise for a host of technologies, including field-effect transistors, chemical catalysis and fluorescent biomedical imaging as well as LEDs and photovoltaic cells. Because nanocrystals have a large surface area relative to their volume, their surfaces play an important role in many physical and chemical processes.
Notably, these surfaces can be modified and functionalized with ligands, which are typically smaller molecules such as long-chain amines, thiols, phosphines and phosphonates. The presence of these ligands changes the nanocrystals’ behaviour and properties. For example, they can make the nanocrystals hydrophilic or hydrophobic, and they can change the speed at which charge carriers travel through them. This flexibility allows nanocrystals to be designed and engineered for specific catalytic, optoelectronic or biomedical applications.
Quantifying ligand density
Previous research showed that the size of nanocrystals affects how many surface ligands can attach to them. The curvature of the crystals can also have an effect. The new work adds to this body of research by exploring the role of nanocrystal shape in more detail.
In their experiments, Jia and colleagues measured the density of OLA ligands on ZnS nanocrystals using three techniques: thermogravimetric analysis-differential scanning calorimetry; 1H nuclear magnetic resonance spectroscopy; and inductively-coupled plasma-optical emission spectrometry. They combined these measurements with semi-empirical molecular dynamics simulations.
The experiments, which are detailed in the Journal of the American Chemical Society, revealed that Zn nanoplatelets with flat basal planes and uniform surfaces allow more ligands to attach tightly to them. This is because the ligands can stack in a parallel fashion on the nanoplatelets, whereas such tight stacking is more difficult on Zn nanodots and nanorods due to staggered atomic arrangements and multistep on their surfaces, Jia tells Physics World. “This results in a lower ligand density than on nanoplatelets,” he says.
The Curtin researchers now plan to study how the differently-shaped nanocrystals – spherical dots, rods and platelets – enter biological cells. This study will be important for improving the efficacy of targeted drug delivery.