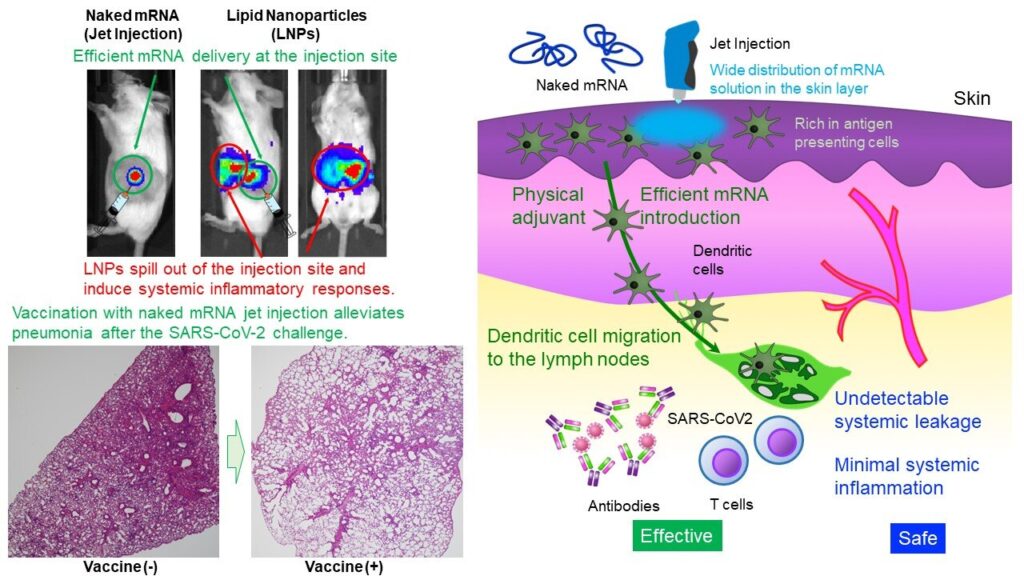
The Uchida Laboratory of Innovation Center of NanoMedicine has demonstrated that intradermal administration of mRNA alone (naked mRNA) without protection by nanoparticles induced robust vaccination against SARS CoV-2, a virus causing COVID-19, in mice and primates. mRNA is highly unstable, generally considered to require a tiny capsule, such as lipid nanoparticles (LNPs), for administration.
The method reported here is the first naked mRNA vaccine demonstrating prevention against SARS-CoV-2. Without using LNPs, which are highly likely to cause systemic adverse events, this vaccine may allow repeated dosing. It is now under development for clinical trials. Detailed research results will be published in Molecular Therapy.
During the COVID-19 pandemic, mRNA vaccines have demonstrated outstanding efficacy, with billions of doses administered worldwide. However, challenges have arisen amidst their rapid development, notably concerning relatively strong adverse reactions, including severe ones, which remain significant issues.
While these adverse reactions may be deemed acceptable for a limited number of doses during a pandemic, a safer platform allowing multiple doses over a lifetime is desirable for ongoing COVID-19 boosters and the extension of mRNA vaccine application to other infectious diseases. Current mRNA vaccines have been associated with adverse reactions, primarily attributed to lipid nanoparticles (LNPs) that carry the mRNA (mRNA cloaked in a lipid coat).
LNPs possess immunostimulatory properties and can spill out of the injection site, leading to systemic inflammatory responses. Nonetheless, LNPs play crucial roles in vaccine efficacy, such as [Function I] preventing mRNA degradation and efficiently delivering mRNA into cells, [Function II] migrating to lymph nodes to deliver mRNA into immune cells, and [Function III] stimulating the immune system through immunostimulatory lipids. The present study aims to obtain these functions without relying on LNPs.
This study provides a straightforward and safe design, the administration of naked mRNA. Regarding [Function II], few immune cells reside in muscle tissue, a current administration site of mRNA vaccines. Therefore, the skin tissue, which is more abundant in immune cells, was targeted.
Furthermore, for [Function I], the research team used a Jet Injector that facilitates mRNA delivery to the skin cells utilizing physical stress induced by jet flow. In a reporter study, Jet Injector improved the mRNA delivery efficiency by more than 100-fold compared to a conventional needle and syringe injection. Also, mRNA stayed at the injection site without detectable systemic leakage.
On the other hand, mRNA-loaded LNPs (mRNA cloaked in a lipid coat) migrated to the liver, spleen, and other systemic organs after intradermal administration, provoking inflammations there. In addition, inflammation at the injection site was very minor in our method, whereas mRNA cloaked in a lipid coat induced infiltration of inflammatory cells and tissue necrosis.
Next, the research team first demonstrated the vaccination ability of naked mRNA using a model antigen. The jet injector drastically improved the efficacy of antibody production to a level comparable to that of mRNA cloaked in a lipid coat at the maximum tolerable doses.
These antibodies combat viruses in the body, preventing infection, but they cannot remove infected cells. On the other hand, cellular immunity removes such diseased cells, playing a critical role in preventing severe diseases. Intriguingly, the naked mRNA vaccine effectively increases the number of immunocytes, such as CD4-positive T cells and CD8-positive T cells.
Then, the research team conducted virus challenge experiments after the naked mRNA vaccination targeting the spike protein of the SARS-CoV-2 virus. The vaccination significantly lowered the amount of virus in the lungs and alleviated lung inflammation compared to an unvaccinated control. This vaccine provided cynomolgus monkeys with vaccine efficacy comparable to that of mice without significant adverse reactions.
The present study also includes mechanistic analyses. Regarding [Function II], the naked mRNA vaccine stayed at the injection site and did not migrate to the lymph nodes. On the other hand, antigen-presenting cells that took up mRNA at the injection site migrated to the lymph nodes, which may contribute to the vaccination efficacy.
Indeed, the vaccine-induced the maturation of the lymph node near the injection site. For [Function III], the Jet Injector caused transient inflammation localized to the injection site, recruiting lymphocytes. Needle and syringe injection of naked mRNA did not induce such an inflammatory response. These results suggest that the immune stimulation by Jet Injector may function as a Physical Adjuvant to enhance the vaccination efficacy. Observed local inflammatory reactions disappeared within a few days.
In conclusion, the naked mRNA vaccine reduces systemic adverse reactions, an issue with mRNA cloaked in a lipid coat, and induces immunity sufficient for the protection from infectious diseases. This is a world-leading achievement in preventing infectious diseases with mRNA alone. Practically, this vaccine may become a platform that allows repeated dosing with minor adverse reactions. Currently further studies are being conducted, with the aim of a clinical trial planned for 2026.
Provided by
Innovation Center of NanoMedicine