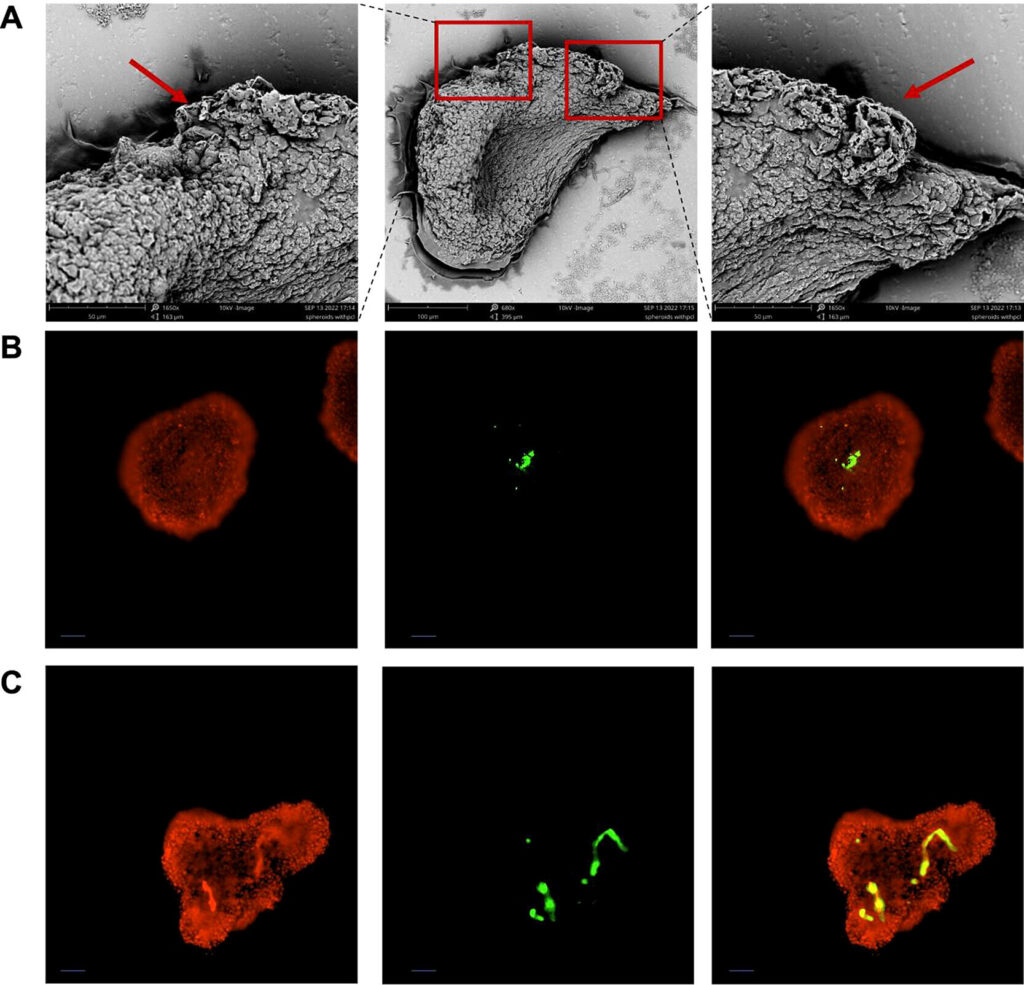
A laboratory-grown mini liver model uniquely created with liver cells and a synthetic nanoscaffold has shown to be effective in mimicking the liver, promising a new and more effective testing method for medicines that is more ethical than animal testing.
The mini liver model, which was created in Dr. Bahijja Raimi-Abraham’s laboratory, uses a novel approach that combines liver cells with synthetic scaffolds. These mini livers, or 3D liver spheroids, are designed to mimic the structure and function of the human liver more accurately than traditional 2D cell culture models. This approach offers a promising alternative to animal models for preclinical drug screening and toxicity testing.
“This research marks a crucial milestone in the pursuit of ethical and effective drug testing methods. By accurately replicating human liver functions, our laboratory-grown mini liver model not only addresses the ethical concerns associated with animal testing but also offers a more reliable platform for evaluating drug safety and efficacy,” said Dr. Raimi-Abraham, Senior Lecturer in Pharmaceutics.
Drug discovery research has used animal models for decades to test the safety of new medical candidates. However, animal models pose significant ethical concerns and practical challenges, including physiological differences between animals and humans, high costs, and tissue availability. Consequently, there is growing interest in developing non-animal testing methods, one of which is using laboratory-grown human cell models.
The liver plays a crucial role in drug development as a major site for drug metabolism. However, drug-induced liver injury (DILI) is a key roadblock as metabolic reactions can result in toxic side effects. This can result in acute liver failure and a frequent factor in drug withdrawals during clinical trials.
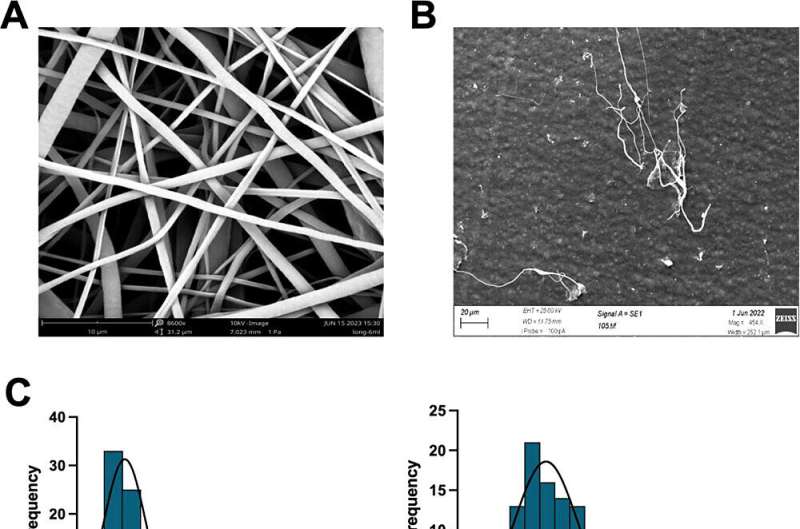
To overcome this barrier, Dr. Raimi-Abraham’s team have designed a new laboratory-grown mini liver model created with liver cells and a synthetic nanoscaffold that effectively mimics liver cells for drug testing. The synthetic nanoscaffold was constructed to provide a supportive structure for the liver cells, creating a “cell soup” that contains the liver cells and the connecting nanoscaffold.
Their results, published in ACS Applied Materials & Interfaces, highlights the success of the new laboratory-grown mini livers, which demonstrated superior cell assembly and liver replication. Further analysis showed that these models exhibited enhanced drug metabolism capabilities compared to other mini liver models that didn’t use the nanoscaffold.
This indicates that the new laboratory-grown mini liver model could have the potential to replace animal testing in drug screening. By addressing the ethical and practical of animal models, including physiological differences, associated high costs and limited tissue availability, this innovative approach shows great potential as a more accurate and ethical alternative.
“This research represents a pivotal moment in my Ph.D. journey, offering both a profound sense of achievement and a glimpse into the future of biomedical innovation. Developing the mini liver model has not only been a testament to the potential of nanotechnology in advancing medical research but also a deeply rewarding experience in overcoming complex scientific challenges,” said Lina Wu, China Scholarship Council Ph.D. Researcher, King’s.
In response to ethical concerns, medical regulators including the US Food and Drug Administration (FDA) and the Medicines and Healthcare products Regulatory Agency (MHRA) have advocated for the increased uptake of non-animal models in drug discovery and development. The FDA Modernization Act 2.0 now permits alternatives to animal testing for drug and biological product applications. Dr. Bahijja Raimi-Abraham and her team believe their laboratory-grown mini livers represent a critical step in this transition.
Beyond mini liver models, The Raimi-Abraham lab aims to apply their nanoscaffold technology to develop models for other organs and create mini-organ models integrated with microbes to model specific infectious diseases such as malaria. Further research will also look more closely at the molecular mechanisms which the nanoscaffolds use to support the cells within the models.