Bacterial biofilm infections are among the leading causes of morbidity and mortality among patients with cystic fibrosis or those with weakened immune systems. Treatment for biofilm infections usually entails intensive antibiotic therapy. There is an urgent need for well-designed agents to achieve in vivo diagnosis and precise anti-biofilm therapy without bacterial resistance. Unfortunately, no effective agent has been designed to perform these tasks adequately.
In a study published in ACS Nano, a research group led by Prof. Chen Xueyuan from Fujian Institute of Research on the Structure of Matter (FJIRSM) of the Chinese Academy of Sciences (CAS) achieved noninvasive phototheranostics in a mice model with Pseudomonas aeruginosa biofilm-induced pulmonary infection.
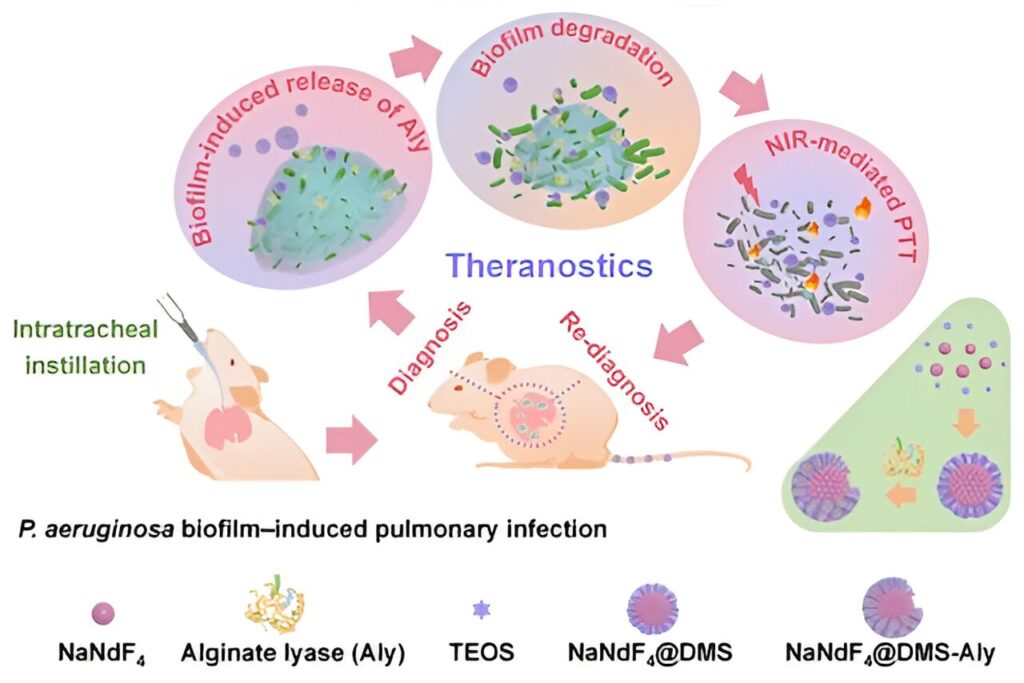
Researchers fabricated novel sunflower-like structured alginate lyase (Aly)-NaNdF4 nanohybrids through an enrichment-encapsulating strategy, which showed an excellent photothermal conversion efficiency, the second near-infrared (NIR-II) luminescence emission, as well as an ideal particle size for delivery to the lung.
When Aly-NaNdF4 nanohybrids were located in biofilm-infected lungs, pH-responsive Aly molecules were released from the mesopores of nanohybrids due to the acidic environment, thus resulting in targeted degradation of biofilms. At the same time, the nanohybrids showed a strong positive charge that enhanced their adhesion to bacteria, which prolonged the stay of nanohybrids in the lung.
By monitoring the luminescence intensity of nanohybrids in the lungs, the extent of infection and therapeutic effectiveness could be evaluated in real time.
Researchers showed that the nanohybrids achieved a strong biofilm eradication by utilizing the synergistic effect of Aly and photothermal therapy. The viability of P. aeruginosa in vitro was reduced by 5.3 log10, achieving a disinfecting effect. The number of bacteria in the lungs of the treated mice was reduced by 94%.
Notably, the nanohybrids were mainly metabolized in the liver and spleen, and could be basically cleared from the body on day eight after intravenous injection, effectively avoiding the potential long-term toxicity of nanomaterials.
This study is an exploration of biofilm-targeted theranostic nanoagents toward internal organ infections based on luminescent lanthanide nanohybrids, which is of great significance to facilitate precision medicine research and clinical practice in the treatment of biofilm-associated infections.