Polymer solar cells are lightweight, flexible solar panels that can be used for wearable devices. However, toxic halogenated processing solvents used during manufacturing of these solar cells have limited their widespread adoption.
Alternatives to halogenated processing solvents are not nearly as soluble, thus requiring higher temperatures and longer processing times. Finding a way to remove the need for the halogenated processing solvents could improve organic solar cell efficiency and make polymer solar cells a viable option for wearable devices.
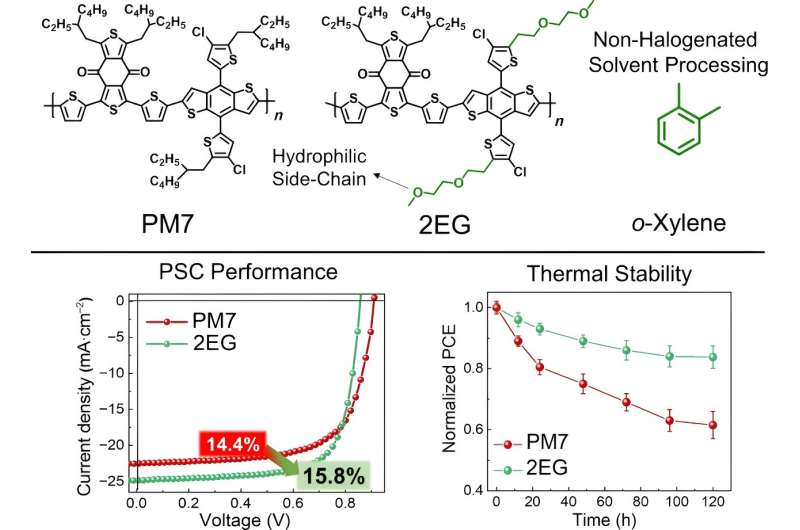
In a paper, published in Nano Research Energy on July 24, researchers outline how improving molecular interactions between the polymer donors and the small molecule acceptors using side-chain engineering can reduce the need for halogenated processing solvents.
“Blend morphology of polymer donors and small molecule acceptors are highly affected by their molecular interactions, which can be determined by interfacial energies between the donor and acceptor materials. When their surface tension values are similar, the interfacial energies and molecular interactions between the donors and the acceptors are expected to be more favorable,” said Yun-Hi Kim, a professor at Gyeongsang National University in Jinju, Republic of Korea.
“To enhance the hydrophilicity of the polymer donors and reduce molecular demixing, side-chain engineering can be a plausible avenue.”
Side-chain engineering is when a chemical group, called a side chain, is added to the main chain of a molecule. The chemical groups in the side chain affect the properties of the larger molecule.
Researchers theorized that adding oligoethylene glycol (OEG)-based side chains would improve the hydrophilicity of the polymer donors thanks to the oxygen atoms in the side chains. A molecule with hydrophilicity is attracted to water. Differences in the hydrophilicity of the polymer donors and the small molecule acceptors can impact how they interact.
With increased hydrophilicity of the polymer donors and improved interactions between them and the small molecule acceptors, non-halogenated processing solvents can be used without sacrificing the performance of the solar cell. In fact, polymer solar cells made with OEG-based side chains attached to a benzodithiophene-based polymer donor had a higher power conversion efficiency at 17.7% compared to 15.6%.
In order to compare results, researchers designed benzodithiophene-based polymer donors with either an OEG side chain, hydrocarbon side chains, or side chains that were 50% hydrocarbon and 50% OEG.
“This elucidated the effect of side-chain engineering on blend morphology and performance of non-halogenated solvent-processed polymer solar cells,” said Kim. “Our findings demonstrate that polymers with hydrophilic OEG side chains can enhance the miscibility with small molecule acceptors and improve power conversion efficiency and device stability of polymer solar cells during non-halogenated processing.”
In addition to improved power conversion efficiency, the polymer solar cells with the OEG-based side chains had enhanced thermal stability. Thermal stability is essential for scaling polymer solar cells, so researchers heated them to 120° Celsius and then compared the power conversion efficiency. After 120 hours of heating, the polymers with the hydrocarbon side chains had only 60% of its initial power conversion efficiency and had irregularities on its surface, while the blend of hydrocarbon and OEG retained 84% of its initial power conversion efficiency.
“Our results can provide a useful guideline for designing polymer donors that produce efficient and stable polymer solar cells using non-halogenated solvent processing,” said Kim.
Other contributors include Soodeok Seo, Jin Su Park, and Bumjoon J. Kim of the Korea Advanced Institute of Science and Technology; Jun-Young Park and Do-Yeong Choi of Gyeongsang National University; and Seungjin Lee of the Korea Research Institute of Chemical Technology.
Provided by
Tsinghua University Press