Most cells in the body send out little messengers called extracellular vesicles that carry proteins, lipids, and other bioactive molecules to other cells, playing an important role in intercellular communication. But healthy cells are not the only ones that rely on extracellular vesicles. Cancer cells do, too. Small extracellular vesicles that are shed from tumor cells contribute to how cancer spreads to healthy tissue.
These small messengers could be a key to developing new cancer-fighting drugs and therapies, but it has been unclear how exactly the recipient cells absorb the extracellular vesicles and their cargo. Recent research used state-of-the-art imaging to observe the uptake of tumor-derived small extracellular vesicles by target cells. The results were published in Nature Communications on March 12, 2025.
“In recent years, extracellular vesicles have attracted attention as a carrier of intercellular signaling. However, the mechanism of their internalization by target cells has not been well understood. We wanted to elucidate the pathway and mechanism of internalization of extracellular vesicles by target cells,” said Kenichi G. N. Suzuki, a professor at the Institute for Glyco-core Research at Gifu University in Gifu and a chief at the Division of Advanced Bioimaging, National Cancer Center Research Institute in Tokyo, Japan.
Researchers focused on small extracellular vesicles derived from two different tumor cell lines. Using single-particle imaging with single-molecule detection sensitivity, a high-tech imaging technique, they were able to categorize the small extracellular vesicles into distinct subtypes. They then tracked the internalization pathway of the extracellular vesicles, or how the vesicles were absorbed into their recipient cells.
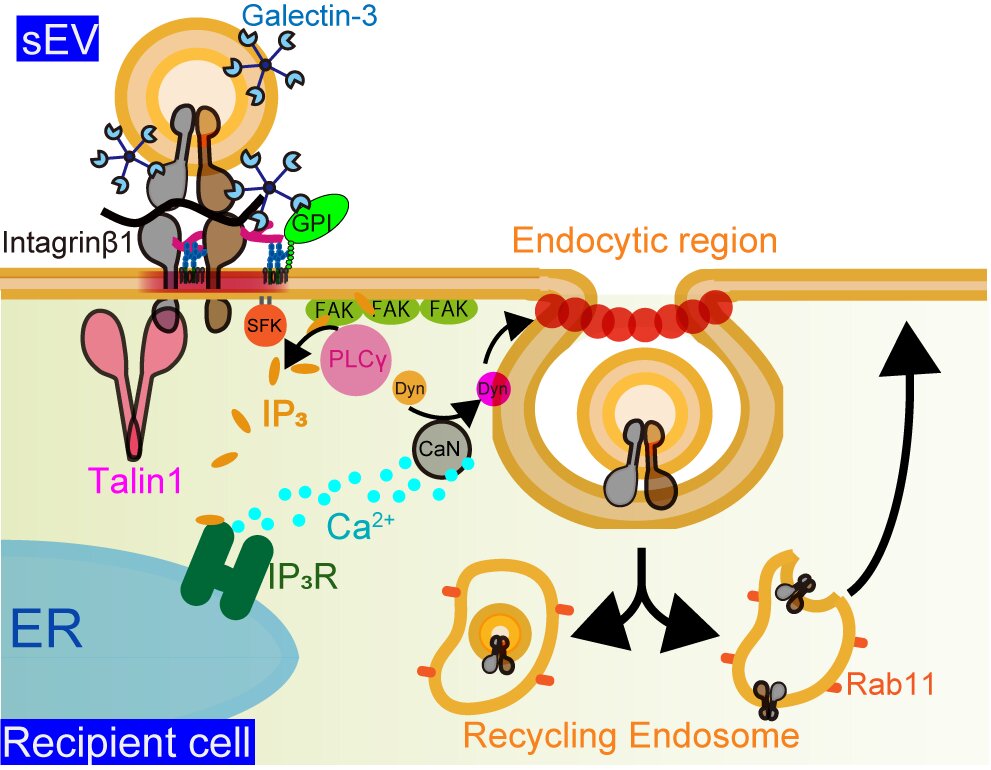
Most small extracellular vesicles were internalized into their target cells through a process called endocytosis. Previously, researchers suspected that the primary mechanism was fusion. During endocytosis, the target cell’s membrane completely surrounds the extracellular vesicle, creating a kind of bubble around the vesicle. This allows it to be absorbed through the membrane so its cargo can be deposited into the target cell.
Researchers did not observe any instances of fusion, where the membrane of the cell and the membrane of the extracellular vesicle fuse together until the vesicle can enter the target cell.
Typically, endocytosis is facilitated with a protein called clathrin, but clathrin was not involved in the absorption of small extracellular vesicles into their target cells. Instead, it was a pair of proteins called galectin-3 and LAMP-2C, which are found on the membrane surface of the small extracellular vesicles.
“Our findings revealed that extracellular vesicles derived from several cancer cells are categorized into distinct subtypes,” said Suzuki. “All the subtypes of extracellular vesicles were primarily internalized by clathrin-independent endocytosis via galectin-3, which was facilitated by an increase in intracellular calcium concentration induced by the binding of extracellular vesicles to the target cells.”
This process where a cell secretes a protein that aids in the absorption into a recipient cell from a different cell line is called paracrine binding or paracrine adhesion signaling. It is in contrast to an autocrine binding, which would be a cell secreting a protein so that it can bind to cells from the same cell line.
By better understanding the process by which small extracellular vesicles are brought into target cells, researchers are hopeful that the vesicles could be used to modify recipient cells and develop new cancer-fighting drugs.
“While extracellular vesicles have been utilized as biomarkers, attempts to use extracellular vesicles as therapeutic agents have begun. Since we have elucidated the mechanism by which extracellular vesicles are taken up by target cells, we expect to contribute to research on extracellular vesicles as therapeutic agents,” said Suzuki.
Other contributors include Koichiro M. Hirosawa, Eriko Yamaguchi, Naoko Komura, Hiromune Ando, and Yasunari Yokota at Gifu University; Yusuke Sato at Tohoku University; Rinshi S. Kasai at the National Cancer Center Research Institute; and Ayuko Hoshino at the University of Tokyo.
Provided by
Institute for Glyco-core Research (iGCORE)