Metallic nanoparticles, consisting of a few to several thousand atoms or simple molecules, are attracting significant interest. Electrodes coated with layers of nanoparticles (nanolayers) are particularly useful in areas such as energy production, serving as catalysts.
A convenient method for producing such layers on electrodes is electrodeposition, the subtle complexities of which have just been revealed by an international team of researchers led by scientists from the Institute of Nuclear Physics of the Polish Academy of Sciences in Krakow. Their paper is published in the journal Nano Letters.
Research on nanoparticles is yielding promising results for technologies related to energy, medicine, and electronics. One of the key challenges is effectively controlling the synthesis and growth of nanostructures.
The team of scientists conducted an advanced experiment demonstrating the electrodeposition process of a platinum-nickel (PtNi) nanolayer on an electrode. Utilizing state-of-the-art imaging techniques, the researchers had a unique opportunity to observe in real-time how structures form at the atomic level, which is a significant step towards better designing materials with precisely controlled properties.
Electrodeposition is a rapid and convenient method for producing nanostructures. It involves immersing an electrode in a metal salt solution, from which the layer is to be grown, followed by applying an appropriate voltage that causes ions near the electrode surface to reduce, initiating layer growth.
To closely examine the process of electrodeposition, transmission electron microscopy (TEM) techniques are essential. TEM allows for imaging materials with sub-angstrom resolution (i.e., less than one ten-millionth of a millimeter) since it uses an electron beam with a much shorter wavelength than visible light. Ideally, it would be possible to observe, in real-time, how nucleation (the initial growth stage where nanoparticle seeds form) and layer growth occur on the electrode.
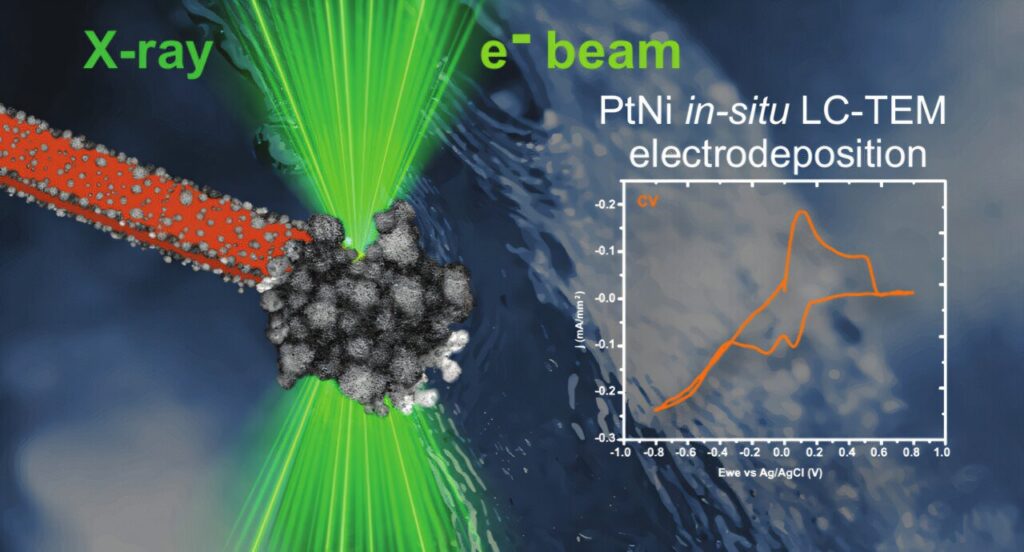
However, TEM imaging comes with certain limitations: the samples need to be as thin as possible and entirely dry. To overcome these challenges and enable the imaging of chemical reactions, the researchers thus utilized a special imaging technique in a liquid cell flow chamber.
“The flow cell consists of two silicon chips equipped with a 50-nanometer-thick SiNx membrane. This membrane is electron-transparent, and an additional electrode is placed on its surface. By applying a voltage, the microscope user can observe how the layer grows on the electrode. Experiments using such a cell require a special holder for flow experiments in the TEM,” explains Prof. Magdalena Parlińska-Wojtan, Ph.D.

Experiments conducted at the Silesian University of Technology using a TEM microscope confirmed that the PtNi layer indeed grows directly on the electrode, providing crucial insights into the fundamentals of the entire process. An alternative mechanism would involve nanoparticles first forming in the electrolyte and then drifting toward the electrode to attach. This effect was also observed, but only in areas illuminated by the beam, due to the fact that the electron beam interacts with water, behaving like a reducing agent.
Subsequent “dry” observations revealed that the layer is actually composed of spherical nanoparticles with diameters of several tens of nanometers. Further magnification of TEM images showed that the surface of these nanoparticles consists of densely branched, fine dendritic structures (multiple branching).
Discover the latest in science, tech, and space with over 100,000 subscribers who rely on Phys.org for daily insights.
Sign up for our free newsletter and get updates on breakthroughs,
innovations, and research that matter—daily or weekly.
“As part of our collaboration with the Fritz Haber Institute of the Max Planck Society in Berlin, we conducted an additional experiment by extending the reaction time and reducing the rate of voltage changes. This allowed us to observe additional effects: the nucleation of individual nanoparticles, which rapidly grow and merge to form a continuous layer.
“During voltage changes in subsequent electrodeposition cycles, the nanoparticles undergo alternating growth and dissolution. However, growth is a faster process than dissolution, which ultimately results in a stable layer,” explains Prof. Parlińska-Wojtan.
As part of the research, another experiment was conducted in liquid environment using a different, but also unique, apparatus: a scanning transmission X-ray microscope (STXM), available at the National Synchrotron Radiation Center SOLARIS in Kraków. During STXM imaging, X-ray radiation is used. The resulting images do not have as high a resolution as the ones from electron microscopy, but they reveal other properties of the materials under study, such as the oxidation states of atoms in nanoparticles.
The result of electrodeposition is not always pure metal; sometimes it is a metal oxide. Depending on whether it is a metal or an oxide (and the oxidation state of the oxide), materials absorb X-ray radiation at different energies. An STXM image taken with the appropriate energy beam allows for a detailed investigation of the produced nanoparticles.
The STXM microscope at the SOLARIS center in Kraków also enabled an experiment in a liquid environment using a flow cell nearly identical to the one used in the TEM. The authors thus performed PtNi electrodeposition inside the STXM and, in real time, investigated the range of X-ray absorption by the nanoparticles. In this way, they determined that the layer actually consists of nickel(II) oxide and metallic platinum.
“Conducting an experiment using microscopic techniques in a liquid environment is quite a challenge. Nevertheless, our team succeeded in producing the expected PtNi layer using two different techniques, and the obtained results were complementary,” says Prof. Parlińska-Wojtan.
“Such research is important for several reasons. The technical reason is that we are still exploring the capabilities and limitations of relatively new, high-end measurement tools. There was also a more important scientific reason: understanding the fundamental factors that govern the synthesis, growth, and properties of nanostructures. This knowledge may help in the future in the fabrication of nanostructured materials tailored better for applications such as fuel cells or medicine.”