Traditional drug delivery methods often lack precision, resulting in low efficacy and causing serious side effects. Novel drug delivery systems (DDSs) address these issues by targeting specific action sites for optimal release, minimizing the impact of the drug on vital tissues, and reducing unwanted side effects.1

Image Credit: Love Employee/Shutterstock.com
DDS can be based on physical mechanisms like osmosis, diffusion, erosion, dissolution, and electro-transport or biochemical methods like monoclonal antibodies, gene therapy, vector systems, polymer-drug adducts, and liposomes.2
Recent advances in nanotechnology have shown that nanoparticles (NPs)—particles smaller than 100 nm in at least one dimension—are promising drug carriers. NPs provide innovative ways for targeted drug delivery due to their enhanced reactive areas and the ability to cross cell and tissue barriers, making them ideal for biomedical use.
Custom-made nanomaterials show great promise in improving disease diagnosis and treatment specificity. Nanotechnology helps overcome the limitations of conventional delivery systems, such as large-scale biodistribution issues and intracellular trafficking challenges, through cell-specific targeting and molecular transport to specific organelles.3
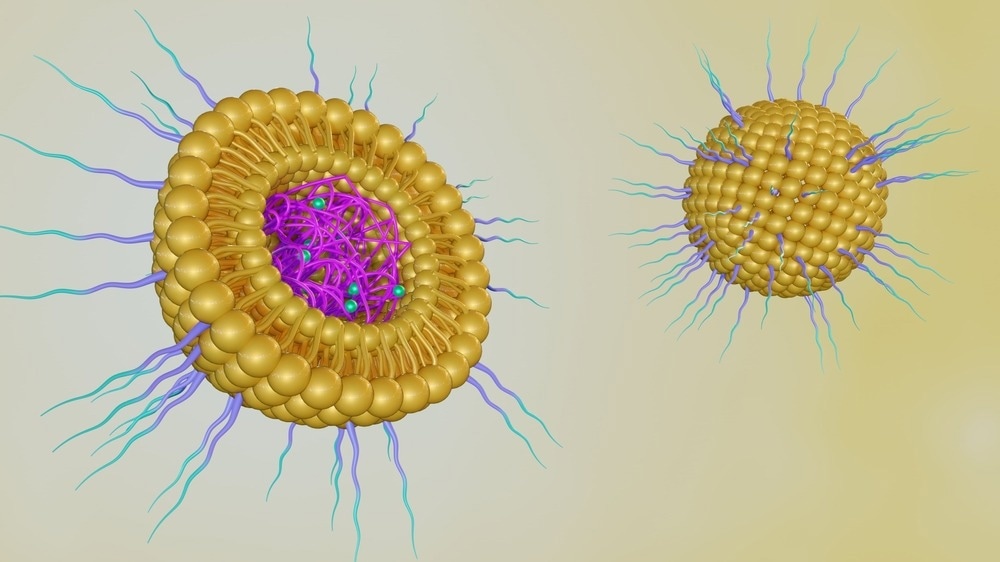
Nanoparticles for Novel Drug Delivery Systems
NPs can carry drugs effectively because of their unique properties, such as enhanced surface area. They are small enough to be absorbed by cells more easily than larger molecules, making them perfect for delivering medicines.3
Drugs can be attached to the surface of these particles, mixed into their structure, or enclosed within them.4 When drugs are covalently linked to the particles, it allows for precise control of how much medicine is delivered.
NPs carrying the drug (nanocarriers) can target specific areas in the body using active methods, like attaching to cell-specific markers, or passive methods, like taking advantage of the leaky blood vessels found in tumors.5 The release of drugs from these particles can be controlled by changes in the body’s environment, such as temperature, pH levels, or specific enzymes.6
To use NPs effectively for drug delivery, it is important to understand how they interact with the body, including which cells they target and how those cells change with disease.
Examples of nanocarriers include liposomes, solid lipid nanoparticles, dendrimers, polymers, silicon or carbon materials, and magnetic NPs.4
NPs made from various materials have their own strengths and weaknesses. For example, polymer-based NPs like lactic-co-glycolic acid and polyethylene glycol are great for use in the body because they are safe and degrade naturally.1 Metal-based NPs, such as those made from gold or iron oxide, have unique optical and magnetic properties useful for imaging, combined treatment, and diagnostic applications.
Lipid-based NPs, like liposomes and solid lipid NPs, resemble cell membranes, aiding their absorption by cells. These NP systems can respond to signals in the body and specific environments to release the drug exactly where needed.7 Designing these nanocarriers effectively requires understanding their functions, targeted locations in the body, retention duration, and handling multiple doses of medication.
Benefits of Using NPs in Drug Delivery
DDS essentially controls how a drug behaves in the body during treatment. NPs have traits that make them ideal for this purpose. They can improve the stability and solubility of the drugs they carry, help transport drugs across cell membranes, and stay in the bloodstream longer, enhancing drug safety and efficacy.
NP-based DDSs reduce many toxic side effects by delivering more drugs to the target site compared to the drug alone. Additionally, they can improve the water solubility of poorly soluble drugs, making them safer without harmful additives.
NPs can also make drugs last longer, require fewer doses, control where the drugs are released, and keep drug levels steady in the body. Other benefits of NPs include being small enough to avoid detection by the immune system, modifying their surface to stay in the bloodstream longer, enhancing their ability to bind to target cells, and allowing multiple drugs to be delivered together in one formulation.
Advancing Disease Treatment with NP-Based DDS
NP-based DDSs are being developed to treat many diseases, including cancer, heart disease, and other chronic disorders. These systems are designed to specifically target cells and tissues by recognizing unique markers that are more pronounced in diseased cells compared to healthy ones.3
For example, by delivering drugs such as paclitaxel or doxorubicin directly to cancerous tissues, NPs can enhance the effectiveness of treatment while minimizing side effects on healthy cells.8
One of the most significant challenges in treating brain cancer and Alzheimer’s disease is the blood-brain barrier, which prevents many drugs from reaching the brain. Researchers have found that NPs can effectively transport drugs across this barrier. For instance, NPs modified with ‘Apolipoprotein E’ successfully delivered loperamide to the brain, showing potential for pain relief.9
Polymeric NPs, made from biodegradable materials like lactic-co-glycolic acid and polyethylene glycol, can be engineered to respond to changes in the body, such as pH, temperature, or enzyme levels.6 This versatility allows them to encapsulate both water-soluble and fat-soluble drugs, making them adaptable for various cancer treatments.
NPs are also being explored in DDS for precision medicine.10 Precision medicine aims to create patient-specific treatments, moving away from the one-size-fits-all approach to improve outcomes. NPs could help more patients qualify for precision medicine by overcoming biological barriers and health conditions that previously excluded them, 10 potentially speeding up clinical trials and allowing more people to benefit from personalized therapies.
NPs are used to deliver treatments for cardiovascular diseases and neurological disorders like Alzheimer’s disease. For instance, they can carry anti-inflammatory agents such as siRNA or curcumin to reduce inflammation and prevent heart disease.11
NPs can also deliver dexamethasone, which stops cell growth and reduces inflammation, directly to affected cells.11 These drug-loaded NPs release medicine slowly over time, helping to stop unhealthy muscle cell growth in blood vessels.12
Future Outlook and Challenges
DDSs are rapidly advancing with the integration of nanoparticles (NPs). These systems aim to personalize treatments based on genetics, biomarkers, and lifestyle factors, promising precise drug delivery to improve treatment effectiveness and reduce side effects.
Combination therapy using these systems shows potential in treating complex diseases like cancer by delivering multiple drugs simultaneously.1 This approach could enhance treatment outcomes and combat drug resistance, a heavily researched topic.
A recent study in Nanotoxicology highlighted the benefits of integrating technologies like biosensors and imaging with nanocarbon-based DDS, enabling real-time monitoring and adjustments for more precise and safer treatments.13
However, these advancements come with significant challenges. One major hurdle is obtaining regulatory approval for these sophisticated systems. Concerns also exist about the materials used in smart DDSs, as noted in a recent report in Emergent Materials.
Some materials could harm cells and tissues, causing unintended side effects.1 Ensuring biocompatibility and safety for human use is crucial for widespread clinical adoption.
Additionally, large-scale and cost-effective manufacturing of these systems poses another challenge. A recent article in ACSMaterials outlines the laboratory and industrial-scale production of lipid-based NPs for drug and gene therapy.
Developing robust production processes is crucial to meet the demand for these advanced therapies.1 Regulatory agencies must also ensure that smart DDSs are safe, effective, and reliable before being approved for patient use.
More from AZoNano: What are the Applications of 3D Printed Graphene?
References and Further Reading
- Boppana, SH., et al. (2024). Current approaches in smart nano‐inspired drug delivery: A narrative review. Health Science Reports. doi.org/10.1002/hsr2.2065
- Bandawane, A., Saudagar, R. (2019). A review on novel drug delivery system: a recent trend. Journal of Drug Delivery and Therapeutics. doi.org/10.22270/jddt.v9i3.2610
- Mitchell, MJ., et al. (2021). Engineering precision nanoparticles for drug delivery. Nature reviews drug discovery. doi.org/10.1038/s41573-020-0090-8
- Wilczewska, AZ., Niemirowicz, K., Markiewicz, KH., Car, H. (2012). Nanoparticles as drug delivery systems. Pharmacological reports. doi.org/10.1016/S1734-1140(12)70901-5
- Raj, S., et al. (2021). Specific targeting cancer cells with nanoparticles and drug delivery in cancer therapy. In Seminars in cancer biology. doi.org/10.1016/j.semcancer.2019.11.002
- Kamaly, N., Yameen, B., Wu, J., Farokhzad, OC. (2016). Degradable controlled-release polymers and polymeric nanoparticles: mechanisms of controlling drug release. Chemical reviews. doi.org/10.1021/acs.chemrev.5b00346
- Alvarez-Lorenzo, C., Concheiro, A. (2014). Smart drug delivery systems: from fundamentals to the clinic. Chemical communications. doi.org/10.1039/C4CC01429D
- Yan, L., Shen, J., Wang, J., Yang, X., Dong, S., Lu, S. (2020). Nanoparticle-based drug delivery system: a patient-friendly chemotherapy for oncology. Dose-Response. doi.org/10.1177/1559325820936161
- Hartl, N., Adams, F., Merkel, OM. (2021). From adsorption to covalent bonding: Apolipoprotein E functionalization of polymeric nanoparticles for drug delivery across the blood–brain barrier. Advanced therapeutics. doi.org/10.1002/adtp.202000092
- Yang, J., Jia, C., Yang, J. (2021). Designing nanoparticle-based drug delivery systems for precision medicine. International Journal of Medical Sciences. doi.org/10.7150%2Fijms.60874
- Mukhtar, M., et al. (2020). Drug delivery to macrophages: A review of nano-therapeutics targeted approach for inflammatory disorders and cancer. Expert Opinion on Drug Delivery. doi.org/10.1080/17425247.2020.1783237
- Joseph, T., et al. (2023). Nanoparticles: Taking a unique position in medicine. Nanomaterials. doi.org/10.3390/nano13030574
- Crous, A., Abrahamse, H. (2021). Innovations in nanotechnology for biomedical sensing, imaging, drug delivery, and therapy. Nanotoxicology. doi.org/10.1021/acs.chemrev.9b00099