Gold particles of the size of billionths of a meter are lethal to cancer cells. This fact has been known for a long time, as has a simple correlation: The smaller the nanoparticles used to fight the cancer cells, the faster they die. However, a more interesting, more complex picture of these interactions is emerging from the latest research, conducted at the Institute of Nuclear Physics of the Polish Academy of Sciences, using a novel microscopic technique.
Smaller kills faster—this is what was previously thought about gold nanoparticles used to fight cancer cells. Scientists thought that small nanoparticles would simply find it easier to penetrate the interior of a cancer cell, where their presence would lead to metabolic disturbances and ultimately cell death.
The reality, however, turns out to be more complex, as demonstrated by research carried out by scientists from the Institute of Nuclear Physics of the Polish Academy of Sciences (IFJ PAN) in Cracow, supported by theoretical analysis performed at the University of Rzeszow (UR) and Rzeszow University of Technology.
“Our institute operates a state-of-the-art medical and accelerator center for proton radiotherapy. So when reports emerged a few years ago that gold nanoparticles could be good radiosensitizers and enhance the effectiveness of this sort of therapy, we started to synthesize them ourselves and test their interaction with cancer cells. We quickly found out that the toxicity of nanoparticles was not always as expected,” says Dr. Joanna Depciuch-Czarny (IFJ PAN), initiator of the research and first author of an article discussing the results, published in the journal Small.
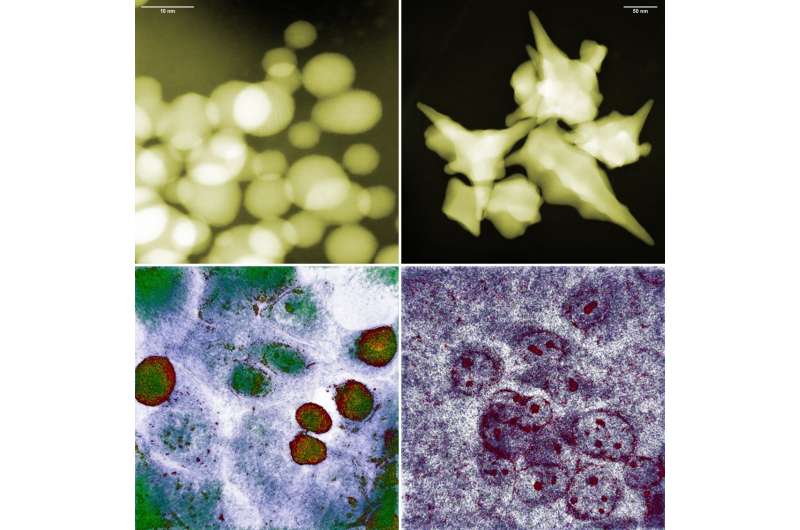
Nanoparticles can be produced using a variety of methods, yielding particles of different sizes and shapes. Shortly after starting their own experiments with gold nanoparticles, the IFJ PAN physicists noticed that biology does not follow the popular rule that their toxicity is greater the smaller they are.
Spherical nanoparticles of 10 nanometers in size, produced in Cracow, turned out to be practically harmless to the glioma cell line studied. However, high mortality was observed in cells exposed to nanoparticles as large as 200 nanometers, but with a star-shaped structure.
Elucidation of the stated contradiction became possible thanks to the use of the first holotomographic microscope in Poland, at IFJ PAN.
A typical CT scanner scans the human body using X-rays, and reconstructs its spatial internal structure section by section. In biology, a similar function has recently been performed by the holotomographic microscope. Here, cells are also swept by a beam of radiation, though not high-energy radiation, but electromagnetic radiation. Its energy is chosen so that the photons do not disturb cell metabolism.
The result of the scan is a set of holographic cross-sections containing information about the distribution of refractive index changes. Since light refracts differently on the cytoplasm and differently on the cell membrane or nucleus, it is possible to reconstruct a three-dimensional image of both the cell itself and its interior.
“Unlike other high-resolution microscopy techniques, holotomography does not require the preparation of samples or the introduction of any foreign substances into the cells. The interactions of gold nanoparticles with cancer cells could therefore be observed directly in the incubator, where the latter were cultured, in an undisturbed environment–what’s more, with nanometric resolution–from all sides simultaneously and practically in real time,” enumerates Dr. Depciuch-Czarny.
The unique features of holotomography allowed the physicists to determine the causes of the unexpected behavior of cancer cells in the presence of gold nanoparticles. A series of experiments was conducted on three cell lines: two glioma and one colon. Among others, it was observed that although the small, spherical nanoparticles easily penetrated the cancer cells, the cells regenerated and even started to divide again, despite the initial stress.
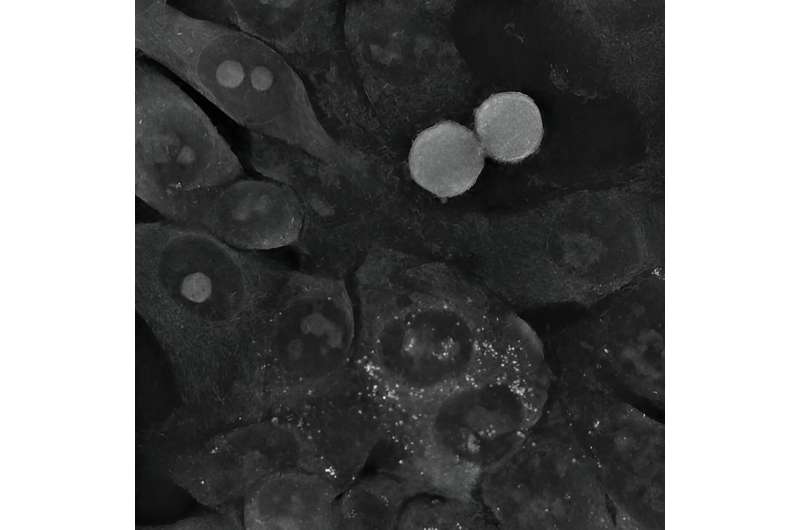
In the case of colon cancer cells, the gold nanoparticles were quickly pushed out of them. The situation was different for the large star-shaped nanoparticles. Their sharp tips perforated the cell membranes, most likely resulting in increasing oxidative stress inside the cells. When these cells could no longer cope with repairing the increasing damage, the mechanism of apoptosis, or programmed death, was triggered.
“We used the data from the Cracow experiments to build a theoretical model of the process of nanoparticle deposition inside the cells under study. The final result is a differential equation into which suitably processed parameters can be substituted—for the time being only describing the shape and size of nanoparticles—to quickly determine how the uptake of the analyzed particles by cancer cells will proceed over a given period of time,” says Dr. Pawel Jakubczyk, professor at the UR and co-author of the model.
He emphasizes, “Any scientist can already use our model at the design stage of their own research to instantly narrow down the number of nanoparticle variants requiring experimental verification.”
The ability to easily reduce the number of potential experiments to be carried out means a reduction in the costs associated with the purchase of cell lines and reagents, as well as a marked reduction in research time (it typically takes around two weeks just to culture a commercially available cell line). In addition, the model can be used to design better-targeted therapies than before—ones in which the nanoparticles will be particularly well absorbed by selected cancer cells, while maintaining relatively low or even zero toxicity to healthy cells in the patient’s other organs.
The Cracow-Rzeszow group of scientists is already preparing to continue their research. New experiments should soon make it possible to extend the model of the interaction of nanoparticles with cancer cells to include further parameters, such as the chemical composition of the particles or further tumor types. Later plans also include supplementing the model with mathematical elements to optimize the efficacy of photo- or proton therapy for indicated combinations of nanoparticles and tumors.

