Venous malformations—tissues made up largely of abnormally shaped veins—are often difficult to treat, especially when located in sensitive areas like the eyes, face, and genitourinary organs. In the worst cases, the lesions are disfiguring and can crush or obstruct surrounding tissues, cause bleeding and clotting, interfere with breathing or vision, or impair circulation.
Katie Ladlie, 25, has been there. Her lesions affect various parts of her body, but most seriously her left leg. As a child, she came to the Vascular Anomalies Center at Boston Children’s Hospital from Missouri for multiple treatments. These helped contain some of her smaller lesions, but not so much her worsening left leg, which became enlarged and painful as blood pooled in the abnormal veins and damaged her knee joint.
Eventually, she could no longer walk and was out of options. At age 12, wanting to play sports, she opted to have her leg amputated at her home hospital in Missouri. With the use of a prosthesis, this would allow her to be more active and mobile.
Creating a new solution for venous malformations
Because of the risk of hemorrhage, malformations like Ladlie’s often can’t safely be treated surgically. Sclerotherapy helped with some of Ladlie’s smaller lesions. But it can be technically different to perform, can also lead to bleeding, and often doesn’t work well, says Steven Fishman, MD, who co-directs the Vascular Anomalies Center and was one of Ladlie’s original doctors. And the available medications only stop malformations from growing, often cause side effects, and must be taken indefinitely.
Ladlie’s case—and others like it—have inspired Boston Children’s researchers to keep searching for new solutions. One, described recently in the journal Nano Letters, looks especially promising.
Several years ago, pediatric intensivist and anesthesiologist Daniel Kohane, MD, Ph.D., and his then-fellow Kathleen “Kate” Cullion, MD, Ph.D., encountered a teen in the ICU with a severe, basketball-sized venous malformation affecting her thigh and leg. Kohane directs the Laboratory for Biomaterials and Drug Delivery (LBDD) at Boston Children’s, and he and Cullion wondered if the lab could help.
They got an idea for a new approach from a second patient with a lymphatic malformation, who had a contrast dye study to better view the vessels. “The dye was still in the malformation two months after the study,” recounts Kohane. “The dye was made up of nanoparticles that zip through normal blood vessels. But the abnormal vessels were leaky and the particles accumulated there. Kate and I said, ‘I wonder if the same thing would happen with venous malformations.'”
This tendency for drugs to diffuse out of malformed, leaky vessels into surrounding tissues could enable a drug to accumulate just where it’s needed, they reasoned. This would increase efficacy, allowing for higher doses yet preventing off-target effects elsewhere in the body.
Nanoparticles plus phototherapy shrink malformations
Kohane and Cullion used mice with bioengineered networks of human vessels that modeled venous malformations, developed in the lab of Juan Melero-Martin, Ph.D. They gave the mice intravenous injections of nanoparticles and tracked the particles’ distribution in the body.
“We proved that you could get nanoparticles to preferentially accumulate in the anomalous vessels,” says Cullion, who is now an attending intensivist and assistant director of the LBDD.
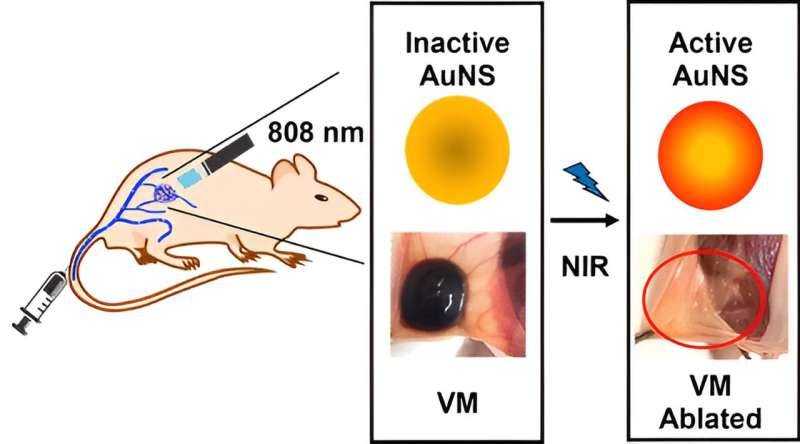
In their latest work, they tested a nanoparticle-based photothermal therapy in a mouse model of venous malformations. First, they injected the mice intravenously with gold nanoparticles, which accumulated in the malformations. Next, they irradiated the nanoparticle-filled lesions with near-infrared light. The irradiated gold particles generated heat, shrinking the malformations dramatically and sometimes eliminating them completely.
Kohane was blown away. “The mice had some venous malformations twice the diameter of a pea and two days later there was nothing.”
A first-in-human trial?
With this proof of principle and early evidence of safety, Kohane, Cullion, and surgical fellow Claire Ostertag-Hill, MD, have filed for a patent on the technology—the first use of nanomedicine to treat vascular anomalies. Kohane thinks it could get to the clinic relatively quickly, as clinical trials are already using a similar approach in cancer.
“I think this treatment will be great for more complex vascular anomalies, or those in difficult locations,” says Ostertag-Hill. “Large anomalies might require several treatments, but at the very least this approach could shrink them and make surgery less risky. In very complex patients, it could be used in conjunction with other procedures.”
Fishman, who as surgeon-in-chief has steered fellows to the Kohane lab, wholeheartedly supports moving forward. “As soon as they have further validation in animals and institutional and regulatory approval, we have plenty of patients who would sign up to be the first human treated, because they have no other good options or other options have failed,” he says. “This work is innovative and will hopefully make a difference.”
Ladlie is hopeful about the research, too. Today, she’s an active member of the U.S. Women’s Sled Hockey team—playing low on the ice, with hockey sticks doubling as ice picks. She has a new job at Spaulding Rehabilitation Hospital where she provides adaptive sports opportunities to people with disabilities.
Though now an adult, she is seeing doctors at Boston Children’s again because of their extensive knowledge of vascular anomalies. “I am so excited with all the new research and education that has been coming out with venous malformations,” she says.