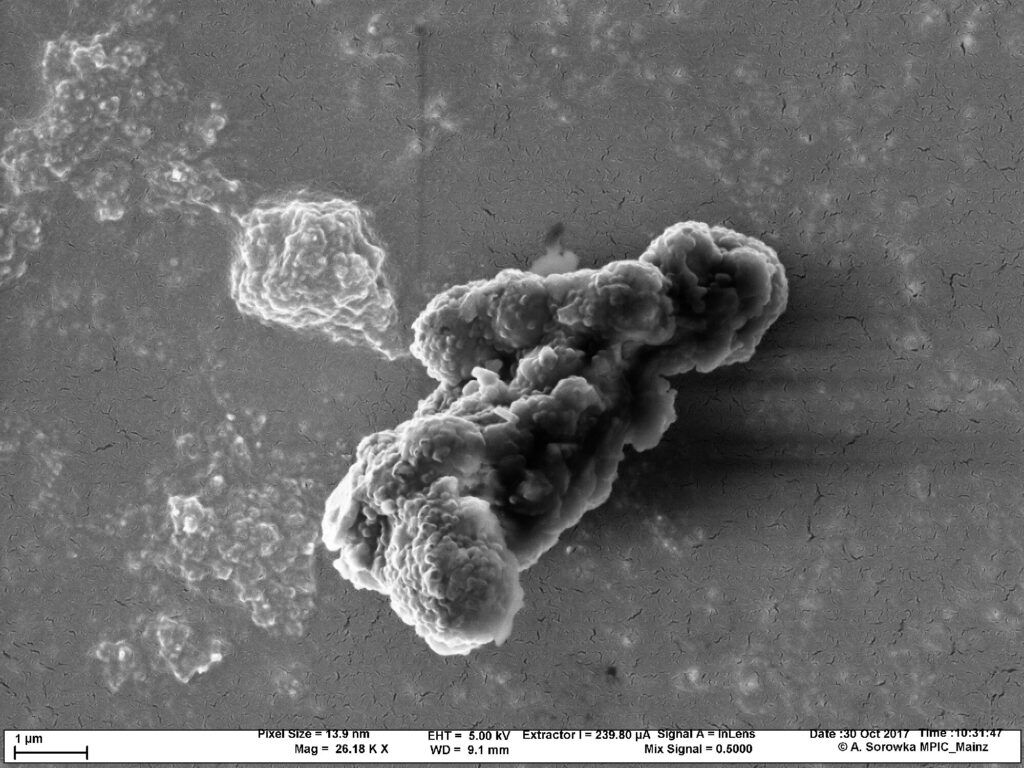
Carbon particles are present in many aspects of our daily lives. Soot, which consists of tiny carbon particles, is generated when energy sources such as oil or wood are not completely burned. Soot particle filters, in turn, remove the nanometer- to micrometer-sized particles from car exhaust fumes with the help of chemical surface reactions.
Carbon particles could be used in industry, because at temperatures above 1,000°C, carbon can be converted with carbon dioxide (CO2) and water into precursors of synthetic fuels. In both applications, chemical reactions occurring on the carbon surface are essential, yet the conditions under which specific reaction pathways dominate are not fully understood.
Carbon particles are degraded by nitrogen dioxide and oxygen
Scientists from the Max Planck Institute for Chemistry (MPIC) can now better explain what happens during the oxidation of carbon nanoparticles in the particulate filter. They examined the tiny soot particles under conditions that are typical for vehicle exhaust gases from diesel engines.
At temperatures ranging from approximately 270°C to 450°C, the carbon interacts with the reactive gases nitrogen dioxide (NO2) and oxygen (O2). The gases oxidize the carbon and thus break it down. The result: The higher the temperature, the faster the carbon mass vanishes. The researchers subsequently entered the experimental data into a kinetic multi-layer model known as KM-GAP-CARBON.
The modeling unveils what happens chemically—at lower temperatures, carbon decomposition is dominated by nitrogen dioxide, whereas at higher temperatures it is dominated by oxygen. This change in dominant reaction pathways is marked by a gradual shift in the activation energy that is necessary for a chemical reaction to take place. Their research is published in Angewandte Chemie International Edition.
Chemical model stems from atmospheric aerosol research
“Our model was originally designed to describe the chemistry of fine-dust particles in the atmosphere, but we found that it also works very well for high-temperature technical applications,” says Thomas Berkemeier, the lead author of the study and research group leader at MPIC.
“Our model helps us to understand why the chemical reaction pathway is influenced by temperature. It also explains a second peculiarity: in the measurements, we observe that the reaction rate is highest in the beginning and at the end of the reaction.”
According to the study, the more reactive carbon atoms on the surface of carbon particles are oxidized and gasified first, leading to an accumulation of less reactive atoms on the surface. This initially leads to a form of passivation of the particles, and the oxidation process slows down.
“Towards the end of the reaction, the ratio of the surface area of the particles to their volume is particularly large, which is why the volume-normalized reaction rate increases sharply again,” explains Berkemeier, who aims to examine the precise structure of the particles in the future using both microscopic and spectroscopic techniques.
Additionally, the chemist and his team are planning further studies on reaction kinetics to explore the effects of various oxidants and conditions.
Basic research contributes to the development of renewable fuels
Ulrich Pöschl, co-author and director at the Max Planck Institute for Chemistry, commented, “Our research not only enhances the understanding of fundamental processes on carbon nanosurfaces. It also opens up new avenues for technological innovations in the environmental and energy sectors, for example, through advancements in carbon capture technologies and to optimize the production conditions in the development of synthetic fuels. The results of decades of basic scientific research thus also contribute to a sustainable development of technology and society in the Anthropocene.”
The term “Anthropocene” refers to the current geological epoch, which is characterized by the rapidly increasing and globally pervasive human influence on planet Earth and has been part of the scientific activities and research at the Max Planck Institute for Chemistry since its discovery by Nobel Prize winner Paul Crutzen.
Provided by
Max-Planck-Institut für Chemie