In recent years, bio-medical engineers have been developing promising techniques that could help diagnose diseases or precisely target specific regions inside the human body. Among these promising therapeutic strategies are methods that rely on the use of nanoparticles (NPs), tiny particles between 1 and 100 nm in size.
These tiny particles could precisely image regions inside the body or deliver drugs to targeted locations. Despite the potential of NPs, for various reasons their therapeutic advantages have so far been limited.
The first main reason is that these tiny particles’ size often limits their ability to enter and penetrate key tissues inside the body, rendering them ineffective for delivering drugs at the necessary concentrations. Moreover, when these particles are introduced into the human body, they are often rapidly captured by the reticuloendothelial system (RES), which is responsible for identifying foreign objects and eliminating them from the bloodstream.
Researchers at the Proteogenomics Research Institute for Systems Medicine recently set out to explore the potential of delivering NPs to lung tissue across cellular barriers, leveraging specialized structures known as the caveolae. Their paper, published in Nature Nanotechnology, demonstrates the feasibility of this approach in a series of initial experiments involving adult rats.
“NPs have tremendous yet unmet clinical potential to carry and deliver imaging and therapeutic agents systemically with tissue precision,” Tapas R. Nayak, Adrian Charastina and their colleagues wrote in their paper.
“But their size contributes to rapid scavenging by the reticuloendothelial system and poor penetration of key endothelial cell (EC) barriers, limiting target tissue uptake, safety and efficacy. We discover the ability of the EC caveolae pumping system to outpace scavenging and deliver NPs rapidly and specifically into the lungs.”
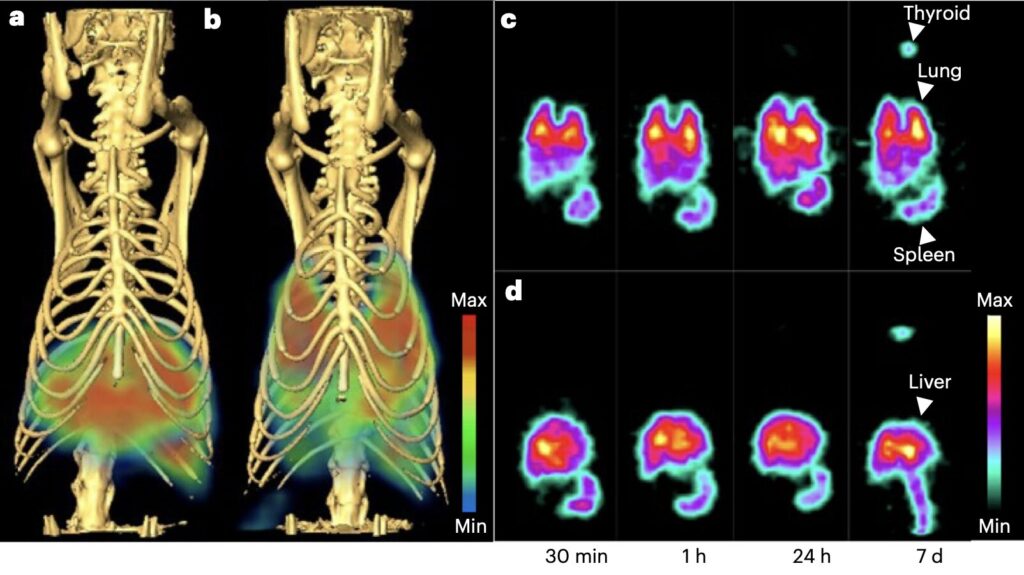
The researchers carried out various experiments where they tried to use metallic and dendritic NPs of different sizes to image and deliver drugs to the lungs of rats. To do this, they employed an alternative approach, which relies on the caveolae pumping system (CPS) to extract the particles from the body, as opposed to the RES.
Caveolae are small invaginations on the membrane of cells that can transport molecules across the endothelial cells lining blood vessels. The CPS is the process via which caveolae can transport these molecules to specific tissues, which the team leveraged as part of their study.
“Gold and dendritic NPs are conjugated to antibodies targeting caveolae of the lung microvascular endothelium,” wrote Nayak, Charastina and their colleagues. “SPECT-CT imaging and biodistribution analyses reveal that rat lungs extract most of the intravenous dose within minutes to achieve precision lung imaging and targeting with high lung concentrations exceeding peak blood levels.”
The initial findings are highly promising, as they found that their proposed method enabled the highly precise imaging of the rats’ lungs and the delivery of drugs to targeted lung tissues using NPs, without the issues typically associated with the expulsion of the particles. New studies could further explore the potential of delivering NPs to the lung by targeting the CPS while also shedding light on factors influencing the effectiveness of this approach, such as the size and shape of the particles used.
“These results reveal how much ECs can both limit and promote tissue penetration of NPs and the power and size-dependent limitations of the caveolae pumping system,” wrote Nayak, Charastina and their colleagues.
“This study provides a new retargeting paradigm for NPs to avoid reticuloendothelial system uptake and achieve rapid precision nanodelivery for future diagnostic and therapeutic applications.”