There is an ever-present struggle to reduce carbon-based energy sources and replace them with low or no-carbon alternatives. The process of splitting water could be the resolution.
Hydrogen production is a simple, safe, and effective method to produce more energy than gasoline can by the simple process of splitting water. Harvesting energy this way, as opposed to relying heavily (or at all) on carbon-based energy sources, is increasingly becoming the standard. Researchers have found a method to use transition metal sulfides, like tin (Sn), cobalt (Co), and iron (Fe) on nickel foam, to develop non-precious metal electrocatalysts for use in cost-effective and environmentally responsible water splitting.
The researchers have published their results in Nano Research Energy.
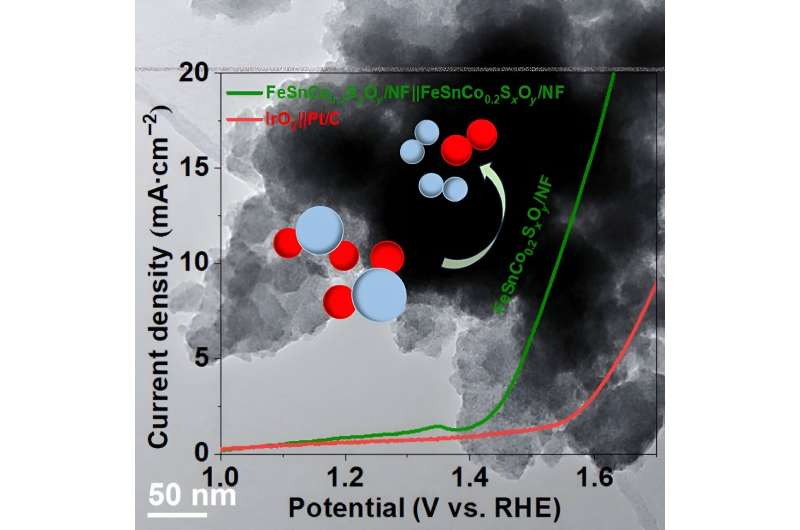
To be successful in this carbon-reducing venture, some reactions need to be stabilized for this process. The star of the study is FeSnCo0.2SxOy/NF, which can act as both an anode and cathode in the process of splitting water at a low voltage.
The two reactions of concern here are oxygen evolution reactions (OER) and hydrogen evolution reactions (HER). OER generates O2 via a chemical reaction from water. HER yields H2 from a two-electron transfer reaction. The resulting H2 is useful as fuel. Using both of these reactions is ideal for creating a bifunctional electrocatalyst. Electrocatalysts can be defined as catalysts (or reaction-starters) that function at electrode surfaces, which are surfaces that can carry an electrical current.
HER has proved to be stable at 55 hours of continuous use and also requires a lower overpotential than OER. Overpotential is the difference in the amount of energy needed for a given catalyst to operate.
Unfortunately, OER stability is not where it needs to be. This is partially due to the extra step involved in the electron transfer but also because the electrolytes they function under are typically harsh. While OER is stable with continuous use of around 70 hours, its activity does decrease with more cobalt content.
“It is pivotal to improve the OER stability of transition metal sulfides so that they can be used as bifunctional HER and OER catalysts for reversible hydrogen fuel cells,” said Jingqi Guan, author and researcher of the study.
OER also has a higher overpotential than HER. With a higher amount of energy needed to induce the catalyst into operation, OER can be more “difficult.” However, the combination of iron, tin, and cobalt on nickel foam boasts some improvement in bifunctional stability and both HER and OER activity.
The combination of these metals and the heterostructural interfaces formed can adjust the distribution of the electrons across the electrolyte surface. “Heterostructural” here refers to a semiconductor that can have an altered chemical composition based on the position the two chemicals are in. In this instance, it is a sulfide/oxyhydroxide duo.
Even distribution of electrons helps to increase the rate of charge transfer throughout the whole structure, which then promotes the transfer of electrons. Due to the nature of this semiconductor, increasing stability naturally would improve overall activity and function.
Overall, these transition metals have a synergistic effect on each other, especially when undergoing HER. This effect makes them ideal candidates for the main challenge proposed by researchers: reducing carbon-based energy sources.
Though the results were very promising, there are always steps that can be made in the future to perfect a process. Finding a catalyst that minimizes the overpotentials can reduce the energy input needed to catalyze the reaction. Additionally, ensuring the electrocatalysts developed are durable enough to be used commercially and can withstand long hours of continuous usage without any ill effects is imperative to the long-term success of the heterostructural interfaces.
Provided by
Tsinghua University Press