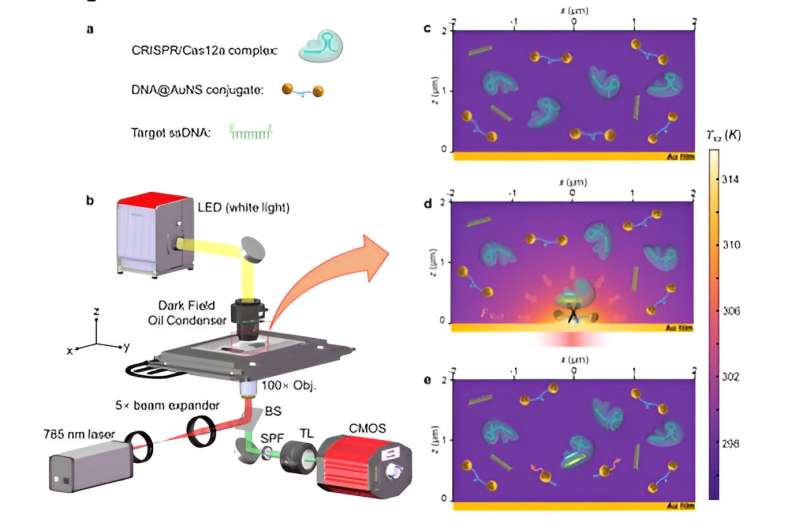
Optothermal nanotweezers, an innovative optical manipulation technique over the past decade, have revolutionized classical optical manipulation by efficiently capturing a broader spectrum of nanoparticles. While this technique has been primarily used for in-situ manipulation of nanoparticles, its potential for identifying bio-nanoparticles remains largely unexplored.
Herein, based on the synergistic effects of optothermal manipulation and CRIPSR-based bio-detection, authors developed CRISPR-powered optothermal nanotweezers (CRONT). Specifically, by harnessing diffusiophoresis and thermo-osmotic flows near the substrate upon optothermal excitation, authors successfully trapped and enriched bio-nanoparticles, including gold nanoparticles, CRISPR-associated proteins, as well as DNA molecules.
In a recent publication published in Light: Science & Applications, a team of scientists led by Professor Jiajie Chen, Zhi Chen, Zhang Han, Yonghong Shao from Shenzhen University, along with their collaborators, Professor Ho-Pui Ho from The Chinese University of Hong Kong have devised an optothermal approach for enhancing CRISPR-based single-nucleotide polymorphism (SNP) detection to achieve single molecule level.
Furthermore, they have introduced a novel CRISPR methodology for observing nucleotide cleavage. Moreover, this innovative approach has endowed optical tweezers with DNA identification ability in aqueous solution, which was unattainable before. Given its remarkable specificity and feasibility for in-situ manipulation and identification of bio-nanoparticles, it is poised to become a universal tool in point-of-care diagnosis, biophotonics, and bio-nanotechnology.
The CRONT can be exquisitely tuned to manipulate bio-nanoparticles and meet the working conditions of CRISPR-based target bio-nanoparticle identification. Specifically, by incorporating optothermal-induced diffusiophoretic force, authors have successfully manipulated bio-nanoparticles, including ssDNA, dsDNA, BSA, Cas12a protein, and DNA functionalized gold nanoparticles.
By incorporating a CRISPR-based DNA biosensing approach, in which the cleavage of a single trapped DNA@Gold-nanoparticle conjugate is interrogated, authors turned this optothermal tweezer into a molecular probe for the in-situ DNA molecules (SARS-CoV-2 or Monkeypox) identification without nucleic acid amplification and achieved detection limits of 25 aM for ssDNAand 250 aM for dsDNA.
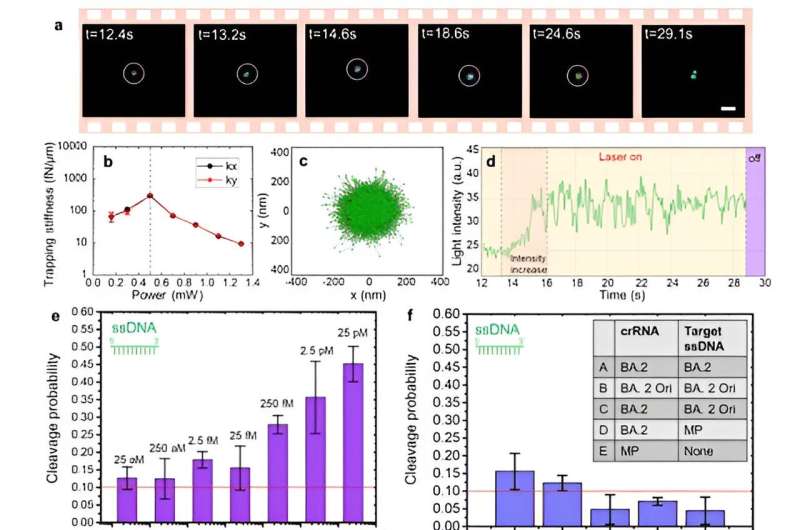
Remarkably, they have demonstrated that these nanotweezers offer single nucleotide polymorphisms (SNPs) identification at ultra-lower detection volumes (10 μL), which play a crucial role in genetic diversity and are associated with various phenotypic traits, including disease susceptibility and drug response. Therefore, this innovation in SNP detection techniques is essential to meet the diverse demands of genomic research and medical applications in the future.
These authors summarized the Work and Outlook of the CRONT as follows:
“CRONT has enabled the immediate implementation of CRISPR-based biosensing within ultra-low detection volume. Optical tweezers are now endowed with DNA identification ability through the CRISPR-based biosensing system. The localized heating properties of CRONT have provided not only an avenue for biomolecule enrichment but also a necessary thermal environment for the cleavage of the CRISPR complex.”
“Further development of this optothermal-based CRISPR bio-detection scheme may involve the utilization of an array of laser heating spots for parallel high-throughput detection, which makes the technique more suitable for quantitative detection and significantly reducing detection time. CRONT may also be employed to guide the CRIPSR/Cas complex to the target DNA and initiate the gene editing process. It also allows the researchers to monitor the gene editing process in real-time at the single-molecule level,” they added.
“We anticipate that such non-contact nanoprobes will contribute to a deeper understanding of various complex biological processes, high lighting optical, thermal, biological similarities at the single-particle level.”