Absorption of light initiates many natural and artificial chemical processes, for example, photosynthesis in plants, human vision, or even 3D printing. Until now, it seemed impossible to control a light-driven chemical reaction at the atomic scale, where only a specific part of one molecule is addressed.
Our international team of scientists has found a solution to that problem by using the light concentration in an atomic-scale volume at the apex of a metallic tip. We were able to induce the switching of two hydrogen atoms in a molecule, a process called tautomerization, and to control the rate of the reaction and its outcome by shining light on different parts of the molecule.
Our research is published in the journal Nature Nanotechnology. In the future, this strategy could be used to synthesize new chemical compounds with properties controlled with atomic precision.
Vision starts with retinal molecules that absorb light hitting the eye. The energy harvested from photons is for a very short time stored in the molecule and can be used to initiate a chemical reaction, in this case, isomerization—a change in the configuration of the atoms and bonds.
The surrounding compounds detect this modification of the retinal shape, which leads to a cascade of events eventually detected by our brain. Other light-induced chemical reactions are important in mechanisms such as photosynthesis in plants or photopolymerization used in both the semiconductor industry for etching and 3D printing.
Even though photoreactions play a determinant role in both nature and industry, studying and controlling such chemical transformations at the most basic unit, that is a single molecule interacting with light, is extremely difficult.
In the usual case, light will interact with many molecules at the same time because the wavelengths of visible photons (400–800 nm) are two orders of magnitude larger than the size of a usual optically active molecule (1–4 nm). Typical optical microscopy is not sufficient to achieve such precision in probing the interaction between light and matter.
Overcoming this issue and being able to play with a photochemical reaction with sub-nanometer precision was the goal of our international team based in France, Czechia, and Germany.
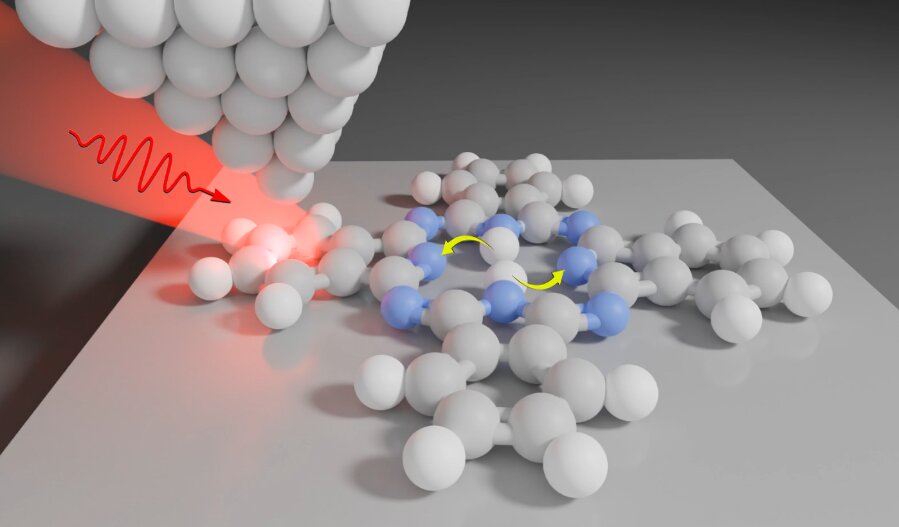
We address this problem by using the ability of very sharp scanning tunneling microscopy (STM) tips, with just a single atom at their apex, to concentrate the laser light down to the sub-nanometer scale. These metallic tips act similarly to usual radio-frequency antennas, except that they work in optical frequencies of the electromagnetic spectrum.
We benefit from this effect and use it to drive a photochemical reaction, which we study not only at a single molecule but also on a subpart of that molecule. By moving the STM tip, we can precisely move the sub-nanometer light spot to different positions above the molecule, and observe how this influences the reaction rate.
This precision is possible because our STM works in ultra-high vacuum, which keeps our system free from any contamination, and in very low temperatures (almost -270°C), so that molecules do not move on the surface.
We studied a reaction called tautomerization, a special form of isomerization in which hydrogen atoms change their positions. In the core of a phthalocyanine molecule, which we used in our study, two hydrogens tautomerize in unison (see the arrows in the figure above).
We control the frequency at which these atoms switch by moving the tip over different parts of the molecule (see the animation) and by changing the color of light that we use for illumination. We can even detect light emitted by our phthalocyanine, which allows us to optically image the molecule with atomic-scale precision and learn more about the tautomerization mechanisms.
Our atomic-scale photochemistry approach is very promising for the future. One can easily imagine using this strategy to synthesize molecules that could not be obtained otherwise. This could be done by moving the tip acting as an atomic-scale light source to, for example, photopolymerize only selected molecular subunits one by one.
This story is part of Science X Dialog, where researchers can report findings from their published research articles. Visit this page for information about ScienceX Dialog and how to participate.