Too often, the lack of clinical trials means that pregnant women suffer because available medications are prescribed off-label for them or not at all. A new study offers proof of concept for the important parameters to develop pregnancy-safe gene therapies.
The power of lipid nanoparticles (LNPs) is more broadly appreciated since the COVID-19 mRNA vaccines were distributed in hundreds of millions of people, including pregnant people. Researchers at Carnegie Mellon University are working to unlock all sorts of new therapies by treating disease at the gene level.
“In thinking about the future of genetic medicine, we would like to understand what could be good for pregnant people as well,” says Kathryn Whitehead.
Whitehead, professor of chemical engineering and biomedical engineering, is among the first to study mRNA delivery during pregnancy.
In a paper published in PNAS, Whitehead provides structural guidance on the design of lipid nanoparticles for safe use during pregnancy. Lipid nanoparticles are the delivery vehicles that bring mRNA into cells.
A lot of the questions surrounding gene therapies in pregnant versus non-pregnant people have to do with the delivery vehicle. It’s not clear if the same delivery vehicle can be used for all or if one needs to be specially developed for safe use during pregnancy.
Whitehead’s research provides insight as to how changes during pregnancy alter nanoparticle behavior compared to non-pregnant people.
The immune system, and its usual response to anything foreign, changes during pregnancy. It is important to understand changes in the immune response to lipid nanoparticles because of potential toxicities and other issues.
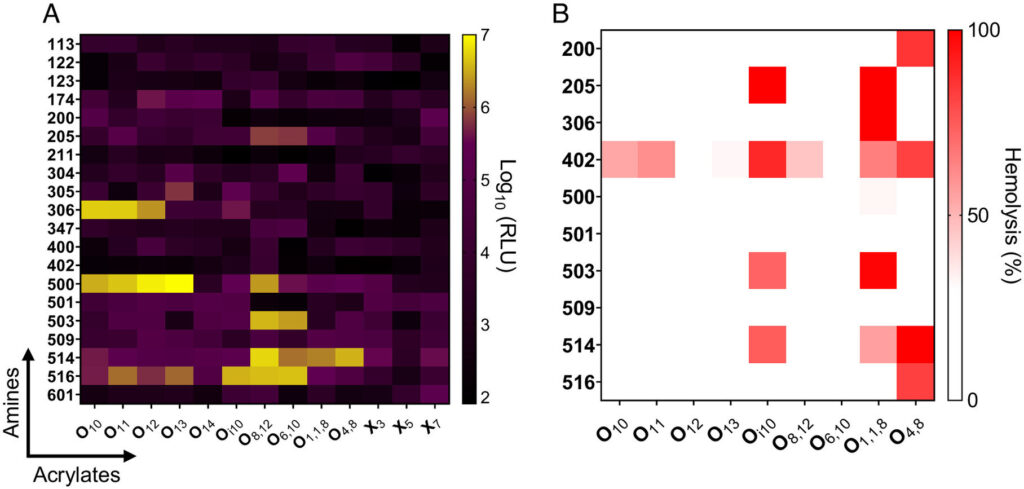
Whitehead’s study shows that lipid nanoparticle effects during pregnancy are chemistry-dependent. The inclusion of different lipids in the nanoparticle alters its chemistry, which in turn changes the way the immune system responds.
In the study, researchers looked at 260 different lipids. They included materials known to work well without causing much of an immune response, materials known to work well and to cause an immune response, and materials known to work poorly.
The study shows that the lipid nanoparticles used in the Whitehead lab do not cross into the fetus. During pregnancy, the body has different barriers to drug delivery. One of the most obvious is the placenta. It is key in providing nutrients to the fetus, while also inhibiting the transport of anything toxic. Most therapies that a woman would need should be kept out of the fetus. Whitehead’s research shows it is possible to deliver mRNA to the placenta without it accumulating in the fetus.
There are a number of diseases where there is dysfunction with the placenta. One of the most common is preeclampsia. “If we can deliver mRNA to the placenta, then it opens up opportunities for therapy and also to explore why these diseases are happening,” says Whitehead.
Researchers also looked at lipid nanoparticles that were shown to be inflammatory in previous research and found that they hindered fetal development. This confirms that delivering an inflammatory lipid nanoparticle during pregnancy is harmful in a quantitative and structurally-dependent way.
There are certain chemistries for which researchers can predict that anything containing that particular structural group will cause a problem. By better understanding structure-function, researchers can more accurately predict what lipids to use in the future.
Collaborators at Magee-Womens Research Institute were instrumental in the early stages of the study. As the research progresses, Whitehead is connecting with new partners there.
Whitehead says this work on mRNA delivery during pregnancy will propel the design of new pregnancy-safe treatments. The findings might lead to better treatments for maternal disorders like preterm birth or preeclampsia, perhaps within the next decade.